- Product Name
- CasNo
- MF
- MW
- Content
- Appearance
- Packing
- Apply
- N,N,N',N'-Tetramethylethylenediamine(TMEDA)
- 110-18-9
- C6H16N2
- 116.206
- Colorless to slightly yellow liquid
Your Location:Home > Products > Catalysts and additives > N,N,N',N'-Tetramethylethylenediamine(TMEDA)

The Eschweiler-Clarke reaction of ethyle...
The synthesis and characterization of 1,...
PdMe2(tmeda) (2a) (tmeda = N,N,N′,N′-tet...
The development of novel antimicrobial a...
More than 80% of the bacterial infection...
The optical spectra and reaction kinetic...
-
N,N,N′,N′-Tetramethylethylenediamine (TM...
B-Triethylboroxin forms colorless 1/1 mo...
The complexation of tetramethylethylened...
-
The present invention pertains to a meth...
The present invention pertains to a meth...
A process for preparing a N-substituted ...
The invention discloses a preparation me...

bis-borane tetramethylethylenediamine

2{((CH3)2NC2H4N(CH3)2)BH2}(1+)*{B12H12}(2-)={((CH3)2NC2H4N(CH3)2)BH2}2{B12H12}


N,N-diethylnmethylamine


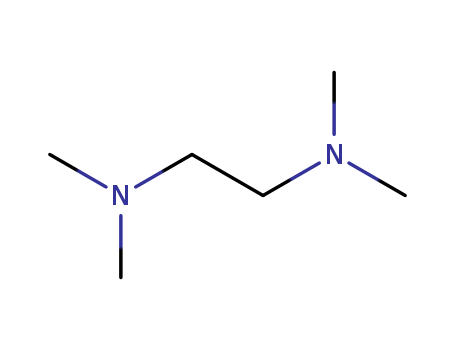
N,N,N,N,-tetramethylethylenediamine


trimethylamine
| Conditions | Yield |
|---|---|
|
In
neat (no solvent);
byproducts: H2; pyrolysis; complete react. at 250°C within 18, at 200°C within 300h;;
|
|
|
In
neat (no solvent);
byproducts: H2; pyrolysis; complete react. at 250°C within 18, at 200°C within 300h;;
|
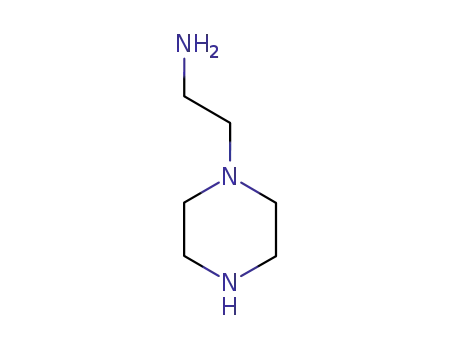
aminoethylpiperazine


formaldehyd

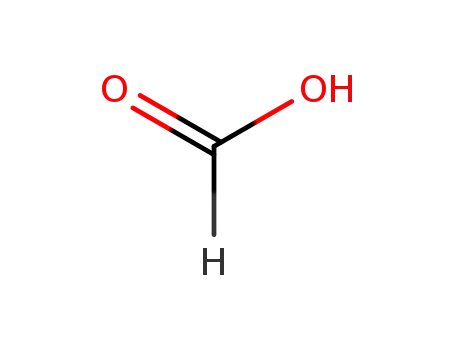
formic acid

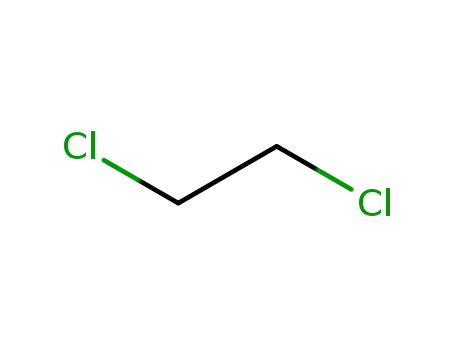
1,2-dichloro-ethane

![1,4-diaza-bicyclo[2.2.2]octane](/upload/2024/12/800544b5-1e7f-490c-8b7c-bf5181efddb0.png)
1,4-diaza-bicyclo[2.2.2]octane


N,N,N,N,-tetramethylethylenediamine

1-<2-(diethylamino)ethyl>-4-methylpiperazine

N,N'-dimethyl-N,N'-bis<2-(4-methyl-1-piperazinyl)ethyl>ethylenediamine
| Conditions | Yield |
|---|---|
|
Multistep reaction. Further byproducts given;
|
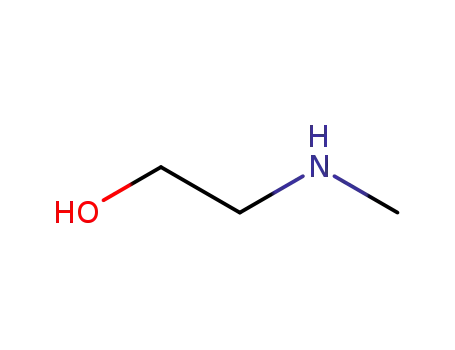
(2-hydroxyethyl)(methyl)amine

formaldehyd
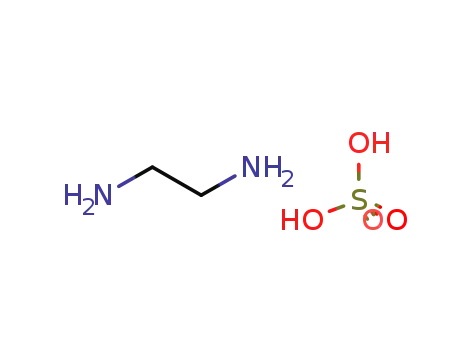
ethylenediammonium sulfate
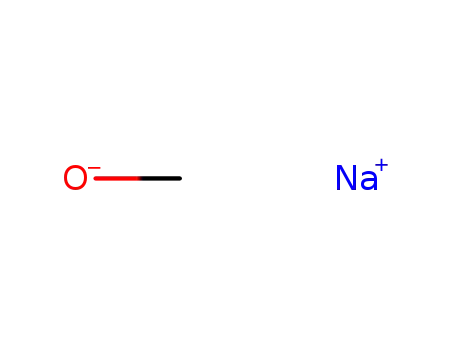
sodium methylate

N,N'-didodecyl-N,N,N',N'-tetramethyl-N,N'-ethanediyldiammonium dibromide
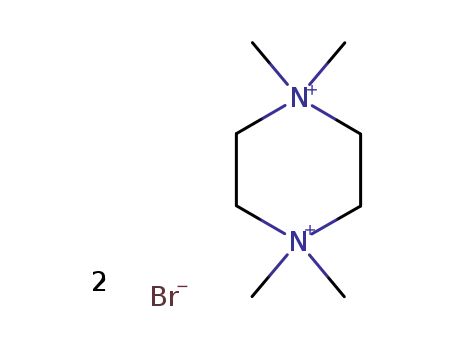
1,1,4,4-tetramethylpiperazinium dibromide
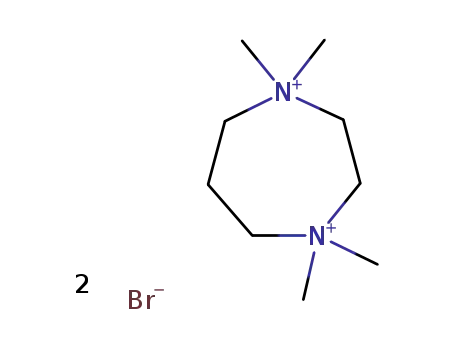
hexahydro-1,1,4,4-tetramethyl-1,4-diazepinium dibromide
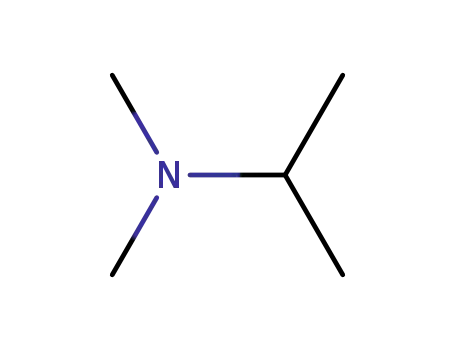
N,N-dimethylisopropyl amine