- Product Name
- CasNo
- MF
- MW
- Content
- Appearance
- Packing
- Apply
- Antioxidant565
- 991-84-4
- C33H56N4OS2
- 588.966
Your Location:Home > Products > Antioxidants > Antioxidant565

|
Flammability and Explosibility |
Notclassified |
|
Synthesis |
By taking 2,6-di-tert-butylphenol as the raw material, and then adding sodium nitrite solution dropwise to form an intermediate product of 2,6-di-tert-butyl-4-nitrosophenol solution, with the added Rayon Nickel is used as a catalyst and carbon monoxide is used instead of hydrogen as a reducing agent to overcome hydrogen as a reducing agent. The pressure in the hydrogenation process is high, which is prone to explosion problems. At the same time, iron crystals are added to remove excess carbon monoxide and avoid the effects of carbon monoxide toxicity. The impurities are removed by filtration, and other by-products will not be formed, which can greatly increase the yield of antioxidant 565, and the product purity is high. |
|
Application |
Antioxidant 565 is a kind of macromolecule, which was a multi-function blocked phenolic antioxidant. Mainly, antioxidant 565 is suitable for the post-processing and stable treatment of unsaturated rubber. It can protect the material from thermal oxidation degradation in the process of production, processing as well as final use. At the same time, antioxidant 565 is a variety of excellent resin antioxidants and photothermal stabilizer, with small addition, low volatility, high color fastness, and can prevent the formation of gel characteristics. Traditionally, antioxidant 565 is a highly effective antioxidant used in a variety of elastomers, including polybutadiene (BR), polyisoprene (IR), latex styrene-butadiene rubber (SBR), nitrile rubber (NBR), carboxylate SBR latex (XSBR), styrene block copolymers such as SBS and SIS. Antioxidant 565 is also used for adhesives (hot melt solvent-based), natural and synthetic viscosifying resins, epdm, ABS, impact resistant polystyrene, polyamide, and polyolefin. |
InChI:InChI=1/C33H56N4OS2/c1-9-11-13-15-17-19-21-39-30-35-29(36-31(37-30)40-22-20-18-16-14-12-10-2)34-25-23-26(32(3,4)5)28(38)27(24-25)33(6,7)8/h23-24,38H,9-22H2,1-8H3,(H,34,35,36,37)
The invention discloses a synthetic meth...
The present invention relates to 4-formy...
2-Hydroxybenzophenone derivatives substi...
2-(2′-hydroxyphenyl)benzotriazoles havin...
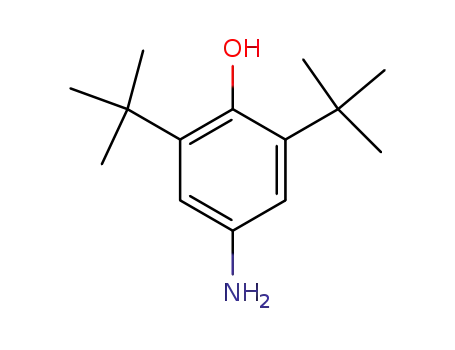
2,6-di-tert-butyl-4-aminophenol

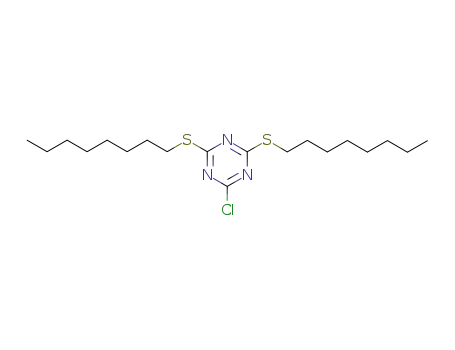
2,4-dioctylthiol-6-chloro-1,3,5-triazine

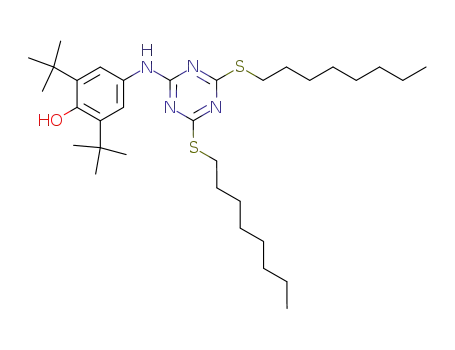
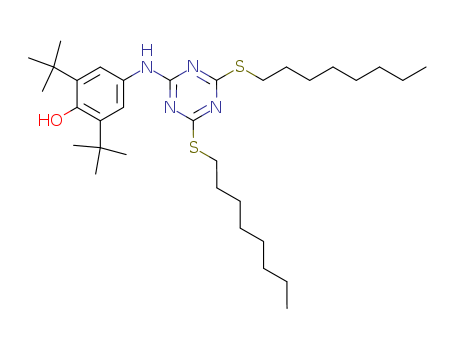
2,6-di-tert-butyl-4-(4,6-bis(octylthio)-1,3,5-triazin-2-ylamino)phenol
| Conditions | Yield |
|---|---|
|
In
toluene;
at 60 ℃;
for 5h;
Time;
|
42.0 g |
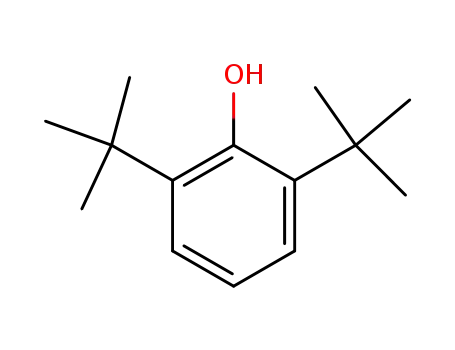
2,6-di-tert-butylphenol


2,6-di-tert-butyl-4-(4,6-bis(octylthio)-1,3,5-triazin-2-ylamino)phenol
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: acetic anhydride; sodium nitrite / methanol; water / 0.5 h / 15 - 30 °C
2: sodium hydroxide; sodium dithionite / water / 1 h / 30 - 65 °C / Inert atmosphere
3: toluene / 5 h / 60 °C
With
sodium dithionite; acetic anhydride; sodium hydroxide; sodium nitrite;
In
methanol; water; toluene;
|

2,6-di-tert-butyl-4-aminophenol

2,4-dioctylthiol-6-chloro-1,3,5-triazine

2,6-di-tert-butylphenol
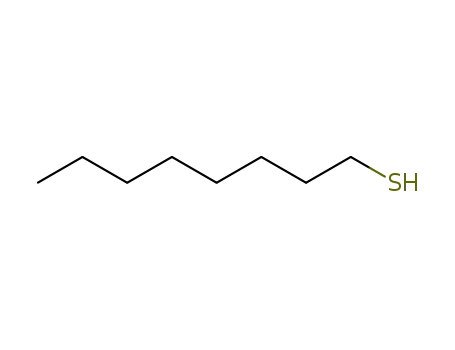
Octanethiol