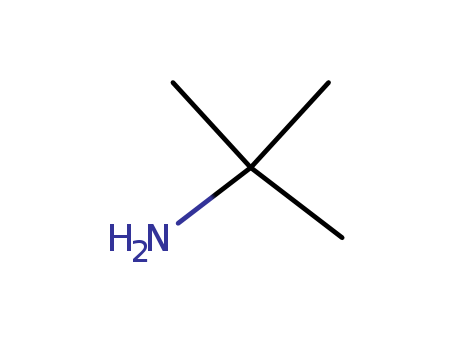
- Product Name
- CasNo
- MF
- MW
- Content
- Appearance
- Packing
- Apply
- tert-Butylamine
- 75-64-9
- C4H11N
- 73.138
- colourless liquid
Your Location:Home > Products > Rubber additives > tert-Butylamine

|
Chemical Description |
Tert-butylamine, 2,6-diisopropylaniline, 2,4,6-trimethylaniline, 2,4,6-tri-tert-butylaniline, and isopropylamine are amines used as solvents. |
|
Production Methods |
tert-Butylamine is manufactured by reacting isobutylamine with sulfuric acid followed by cyanide to tert-butylformamide. Hydrolysis yields t-butylamine. It is used as a solvent and in organic syntheses. |
|
Air & Water Reactions |
Highly flammable. May be sensitive to air . Soluble in water. |
|
Reactivity Profile |
tert-Butylamine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated in combination with strong reducing agents, such as hydrides. Undergoes highly exothermic reaction with 2,2-dibromo-1,3-dimethylcyclopropanoic acid [J. Chem. Soc., 1, 1979, 2324]. |
|
Health Hazard |
Inhalation causes irritation of nose, mouth, and lungs. Ingestion causes irritation of mouth and stomach. Contact with liquid causes severe irritation of eyes and moderate irritation of skin. |
|
Chemical Reactivity |
Reactivity with Water No reaction; Reactivity with Common Materials: Liquid will attack some plastics; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Flush with water; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent. |
|
Safety Profile |
Poison by ingestion. Moderately toxic to humans by inhalation. A corrosive liquid. See also n-BUTYLAMINE and AMINES. Very dangerous fire hazard when exposed to heat or flame. Very exothermic reaction with 2,2-dibromo-l,3 dimethylcyclopropanoic acid. To fight fire, use alcohol foam. When heated to decomposition it emits toxic fumes of NOx. |
|
Purification Methods |
Dry it with KOH or LiAlH4, and/or distil it from CaH2 or BaO. [Beilstein 4 IV 657.] |
|
Definition |
ChEBI: Tert-butylamine is a primary aliphatic amine that is ethylamine substituted by two methyl groups at position 1. It is a conjugate base of a tert-butylammonium. |
|
General Description |
Tert-butylamine appears as a clear colorless liquid with an ammonia-like odor. Flash point 70°F. Less dense (at 6.2 lb / gal) than water. Vapors heavier than air. Toxic oxides of nitrogen produced during combustion. |
InChI:InChI=1/C4H11N/c1-4(2,3)5/h5H2,1-3H3/p+1
-
The number and strength of Broensted aci...
Reaction of [(η5-C5H4Me) 4Fe4(HCCH)(HCC-...
A shaped binderless and two binder-conta...
The reaction between 2-methylpropene and...
-
An activated carbon was functionalized b...
Zeolite beta, an important catalytic mat...
The 3d-metal mediated nitrene transfer i...
Disclosed is a process (100) for convers...
The kinetics of quinuclidine displacemen...
An efficient and chemoselective C(sp2)-N...

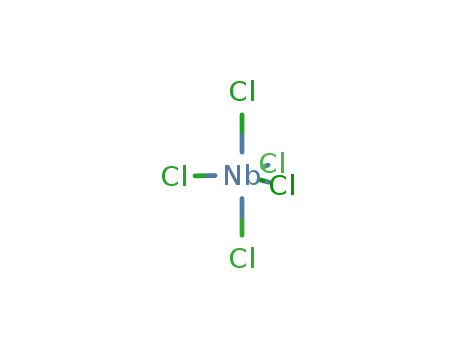
niobium pentachloride

tert-butylamine
| Conditions | Yield |
|---|---|
|
|
79% |

ethyl N-tert-butylcarbamate

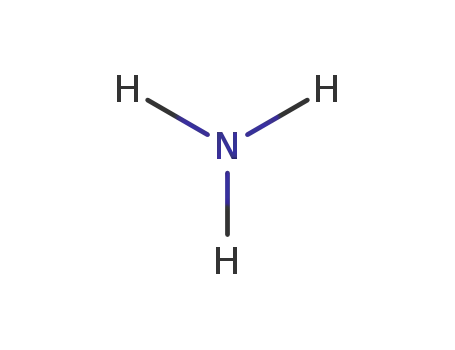
ammonia

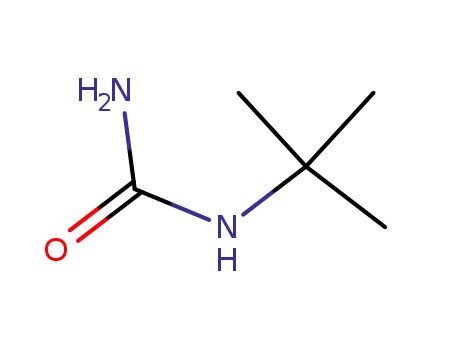
1,1-dimethylethylurea

tert-butylamine

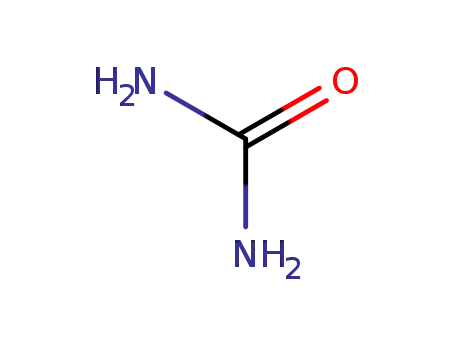
urea

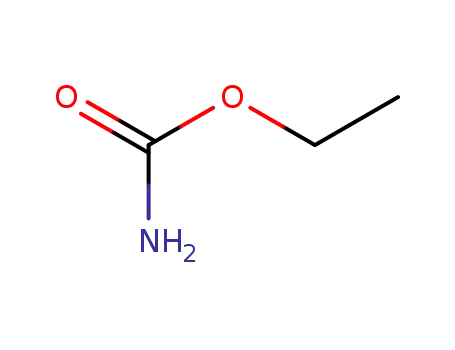
urethane
| Conditions | Yield |
|---|---|
|
at 180 ℃;
|
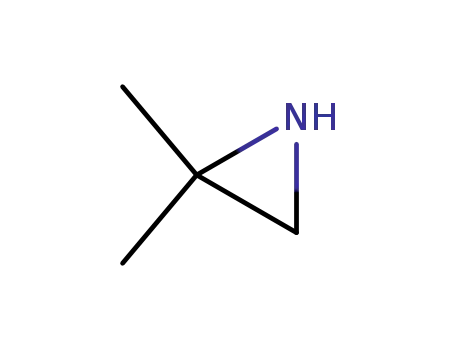
2,2-dimethylaziridine
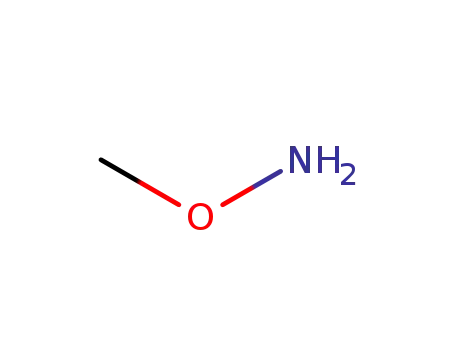
O-Methylhydroxylamin
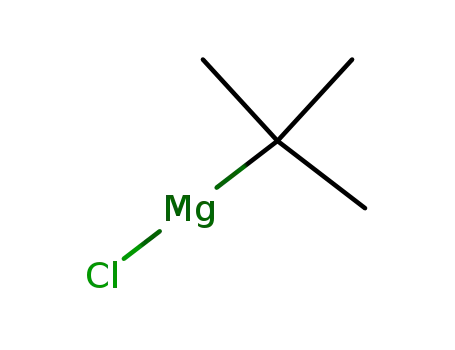
tert-butylmagnesium chloride
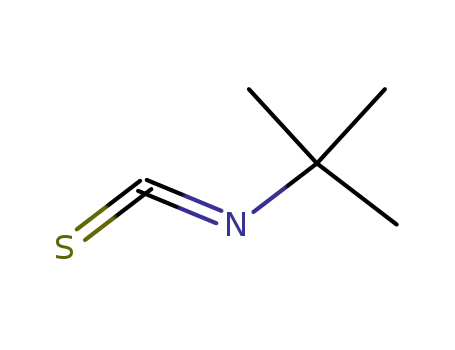
tert-butyl isothiocyanate
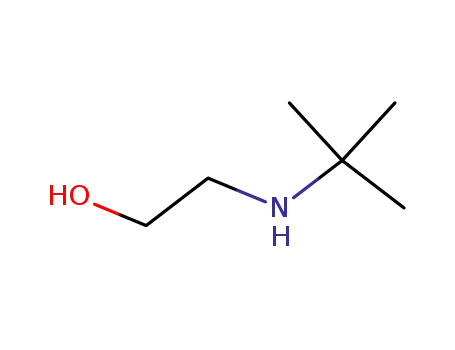
2-[(1,1-dimethylethyl)amino]-ethanol
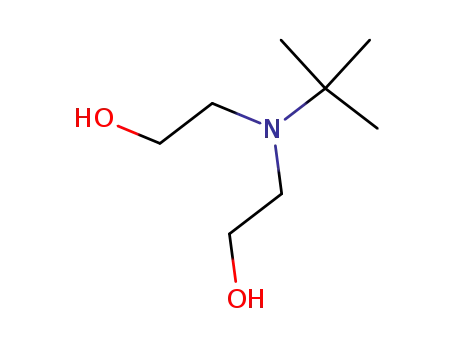
N-tert-butyldiethanolamine
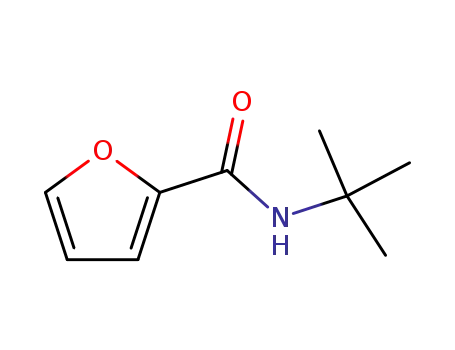
furan-2-carboxylic acid tert-butylamide
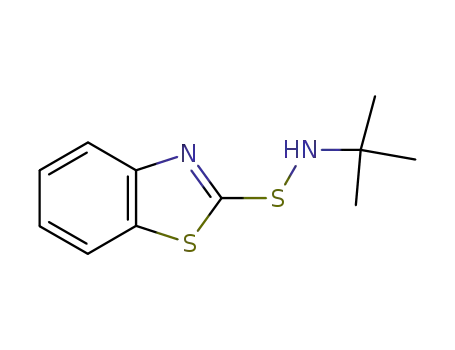
N-(tert-butyl)benzothiazole-2-sulfenamide