- Product Name
- CasNo
- MF
- MW
- Content
- Appearance
- Packing
- Apply
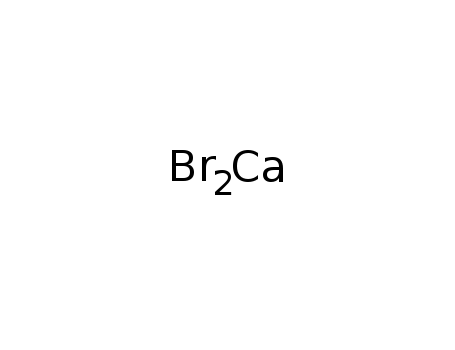
- Calcium bromide
- 7789-41-5
- CaBr2
- 199.886
- white powder
Your Location:Home > Products > Water treatment > Calcium bromide

|
Preparation |
CaBr2·2H2O has a melting point of 38°C where it dissolves in its own waters of hydration. Its density is 2.290 g/cm3. It can be prepared by reaction in solution with HBr on the carbonate: CaCO3 (solid)+HBr (liq)→CaBr2 (liq)+ CO2 (gas) An improved method for producing calcium bromide has been developed by reacting hydrogen bromide with calcium hydroxide in the presence of water. |
InChI:InChI=1/2BrH.Ca/h2*1H;/q;;+2/p-2
A study of binary, CaBr2-CaHBr system wa...
Abstract: Molar heat capacities of two h...
It is already a generally accepted opini...
A study on pseudo-binary CaCl2-CaBr2 sys...
For the low pressure ammonia synthesis (...
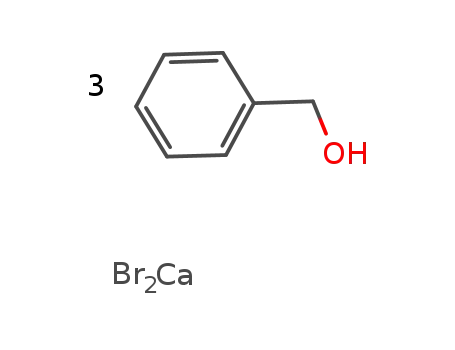
calcium bromide * 3 benzyl alcohol

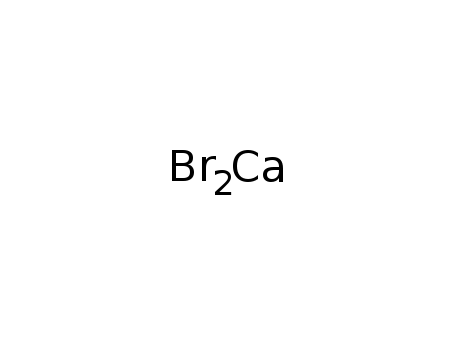
calcium bromide

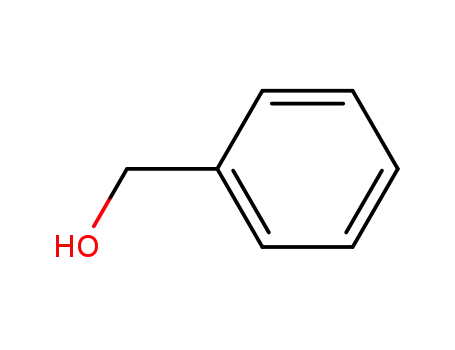
benzyl alcohol
| Conditions | Yield |
|---|---|
|
In
not given;
decomposition at touching the satd. solution at 32°C in its components;;
|
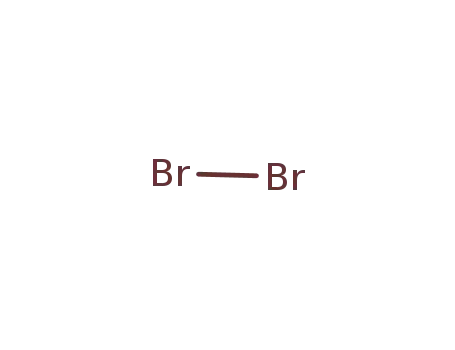
bromine

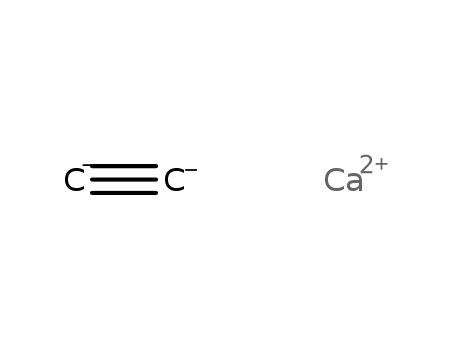
calcium carbide


calcium bromide
| Conditions | Yield |
|---|---|
|
In
neat (no solvent);
passing of a mixture of Br2 and air over CaC2 forms CaBr2 at heating;;
|
|
|
In
neat (no solvent);
byproducts: C2Br6; powdered CaC2 (63%) and dry Br2 in closed tube, 5 month at ambient temperature;;
|
>99 |
|
In
neat (no solvent);
byproducts: C2Br6; touchment of powdered CaC2 and liquid Br2 for some months at normal temperature under formation of CaBr2 and C2Br6;; not isolated;;
|
|
|
In
neat (no solvent);
byproducts: C; formation of CaBr2 and C in a closed tube at 100°C;;
|
|
|
In
neat (no solvent);
byproducts: C; longer heating on boiling water bath;;
|
|
|
In
neat (no solvent);
passing of a mixture of Br2 and air over CaC2 forms no CaBr2 in coldness;;
|
0% |
|
In
neat (no solvent);
passing of a mixture of Br2 and air over CaC2 forms CaBr2 at heating;;
|
|
|
In
neat (no solvent);
byproducts: C2Br6; touchment of powdered CaC2 and liquid Br2 for some months at normal temperature under formation of CaBr2 and C2Br6;; not isolated;;
|
|
|
In
neat (no solvent);
byproducts: C; formation of CaBr2 and C in a closed tube at 100°C;;
|
|
|
In
neat (no solvent);
passing of a mixture of Br2 and air over CaC2 forms no CaBr2 in coldness;;
|
0% |
|
In
neat (no solvent);
reaction at 350°C;;
|
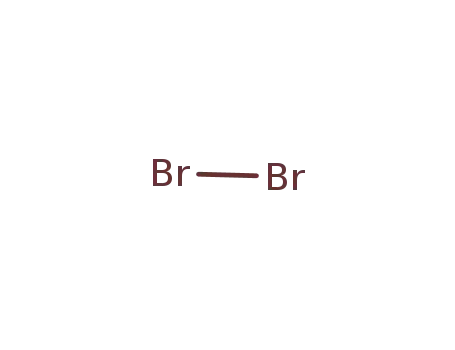
bromine

calcium
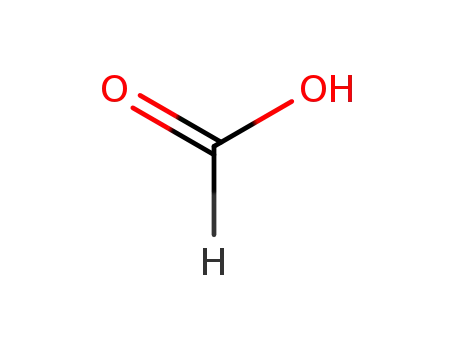
formic acid
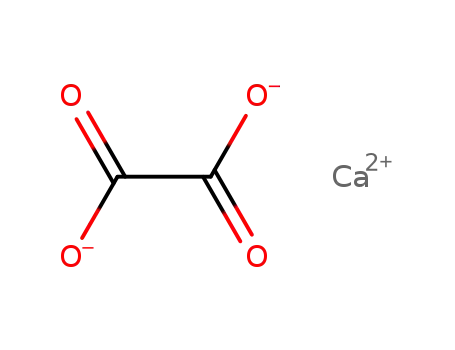
calcium(II) oxalate
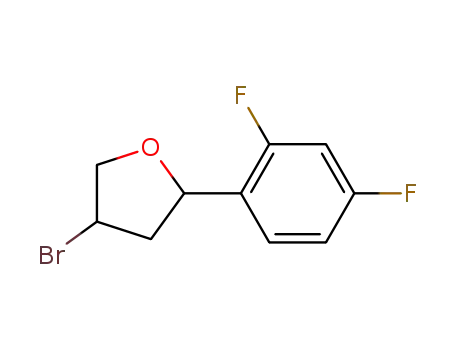
(+/-)-4-Bromo-2-(2,4-difluorophenyl)tetrahydrofuran

potassium bromide
sodium bromide

lithium bromide