- Product Name
- CasNo
- MF
- MW
- Content
- Appearance
- Packing
- Apply
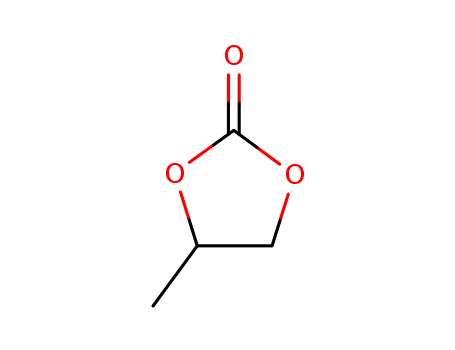
- Propylene carbonate (PC)
- 108-32-7
- C4H6O3
- 102.09
- colourless liquid
Your Location:Home > Products > Electronic Chemicals > Propylene carbonate (PC)

|
Production Methods |
Propylene carbonate may be prepared by the reaction of sodium bicarbonate with propylene chlorohydrin. |
|
General Description |
Propylene carbonate is a cyclic carbonate that is commonly used as a solvent and as a reactive intermediate in organic synthesis. It is being considered as a potential electrochemical solvent due to its low vapor pressure, high dielectric constant and high chemical stability.Propylene carbonate can be synthesized from propylene oxide and CO2. Optically active form of propylene carbonate can be prepared from the reaction between CO2 and racemic epoxides. Decomposition of propylene carbonate on the graphite electrode in lithium batteries results in the formation of a lithium intercalated compound. |
|
Flammability and Explosibility |
Notclassified |
|
Pharmaceutical Applications |
Propylene carbonate is used mainly as a solvent in oral and topical pharmaceutical formulations. In topical applications, propylene carbonate has been used in combination with propylene glycol as a solvent for corticosteroids. The corticosteroid is dissolved in the solvent mixture to yield microdroplets that can then be dispersed in petrolatum.Propylene carbonate has been used as a dispensing solvent in topical preparations. Propylene carbonate has also been used in hard gelatin capsules as a nonvolatile, stabilizing, liquid carrier. For formulations with a low dosage of active drug, a uniform drug content may be obtained by dissolving the drug in propylene carbonate and then spraying this solution on to a solid carrier such as compressible sugar; the sugar may then be filled into hard gelatin capsules Propylene carbonate may additionally be used as a solvent, at room and elevated temperatures, for many cellulose-based polymers and plasticizers. Propylene carbonate is also used in cosmetics. |
|
Safety |
Propylene Carbonate is listed in The Design for the Environment (DfE) Safer Chemistry Program by the EPA as a Solvent category and indicated by the Green circle, meaning the chemical has been verified to be of low concern for human and environmental health based on experimental and modeled data.In animal studies, propylene carbonate was found to cause tissue necrosis after parenteral administration.(mouse, oral): 20.7 g/kg(mouse, SC): 15.8 g/kg(rat, oral): 29 g/kg(rat, SC): 11.1 g/kg |
|
storage |
Propylene carbonate and its aqueous solutions are stable but may degrade in the presence of acids or bases, or upon heating; Store in a well-closed container in a cool, dry place. |
|
Purification Methods |
It is manufactured by reaction of 1,2-propylene oxide with CO2 in the presence of a catalyst (quaternary ammonium halide). Contaminants include propylene oxide, carbon dioxide, 1,2-and 1,3-propanediols, allyl alcohol and ethylene carbonate. It can be purified by percolation through molecular sieves (Linde 5A, dried at 350o for 14hours under a stream of argon), followed by distillation under a vacuum. [Jasinski & Kirkland Anal Chem 39 163 1967.] It can be stored over molecular sieves under an inert gas atmosphere. When purified in this way it contains less than 2ppm of water. Activated alumina and dried CaO have also been used as drying agents prior to fractional distillation under reduced pressure. It has been dried with 3A molecular sieves and distilled under nitrogen in the presence of p-toluenesulfonic acid, then redistilled and the middle fraction collected. [Beilstein 19 III/IV 1564, 19/4 V 21.] |
|
Incompatibilities |
Propylene carbonate hydrolyzes rapidly in the presence of strong acids and bases, forming mainly propylene oxide and carbon dioxide. Propylene carbonate can also react with primary and secondary amines to yield carbamates. |
|
Regulatory Status |
Included in the FDA Inactive Ingredients Database (topical ointments). Included in the Canadian List of Acceptable Nonmedicinal Ingredients. |
InChI:InChI:1S/C4H6O3/c1-3-2-6-4(5)7-3/h3H,2H2,1H3
(Chemical Equation Presented) Sidesteppi...
A series of new half-sandwich titanocene...
Reported is the application of ZIF-90, w...
The commercial production of glycerol ha...
Organocatalysis represents a promising f...
Three organozinc complexes have been syn...
A diamine-bis(phenolate) chromium(iii) c...
Poly(ethylene glycol) (PEG) in this work...
We report here a convenient pathway for ...
Polyols, which can be obtained readily f...
An efficient rare earthmetal complex-cat...
The glycerol carbonate synthesis from gl...
The synthesis of propylene carbonate fro...
The synthesis of cyclic carbonates from ...
Electrochemically activated CO2 reacts, ...
The catalytic alkoxycarbonylation of 1,2...
A highly simple, economical, and selecti...
An efficient, convenient, and environmen...
A series of graphitic carbon nitride mat...
Abstract A series of ZnCl2/mp-C3N4 catal...
Excellent activity and selectivity in th...
Catalytic activity of electrochemically ...
Carbon dioxide (CO2) is a nontoxic and i...
The present protocol involves highly eff...
A practical, safe, and highly efficient ...
We report the first example of a regiore...
The synthesis of propylene carbonate (PC...
-
An environmentally benign catalytic syst...
We have demonstrated the design of a nov...
Catalyzed by ytterbium(III) triflate, as...
In this work, the cycloaddition reaction...
Zn/Mg catalysts with different atomic ra...
Copolymerization of carbon dioxide with ...
-
Three complexes [Fe(L)2](ClO4)2 (1)、[Ni(...
The development of stable and efficient ...
The effect of catalyst liophilicity is s...
Bimetallic bis-urea functionalized salen...
The reaction between CO2 and propylene o...
Mesoporous carbon nitrides (MCN) were pr...
2,2-Dibutyl-1,3,2-dioxastannolans react ...
(equation presented) A new highly select...
One-pot synthesis of well-defined bio-re...
Copper halides, CuX2 (X = Cl, Br), promo...
A novel variety of ionic liquids based o...
The effective conversion of carbon dioxi...
In this study, boiler ash containing pot...
New neutral and cationic chromium(III) c...
Polymer synthesis with CO2 as a C1 chemi...
CO2is the most influential greenhouse ga...
A method for sustainable fixation of CO2...
The invention relates to the field of me...
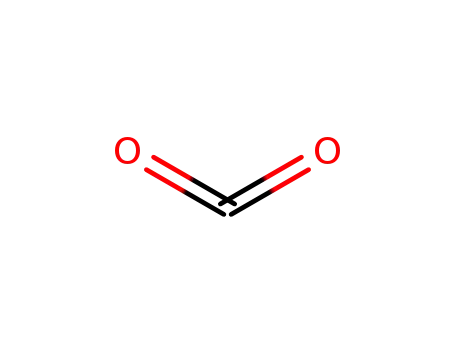
carbon dioxide

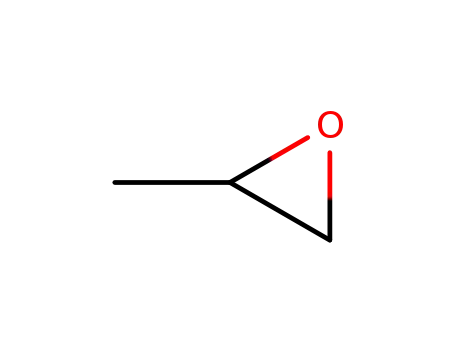
methyloxirane

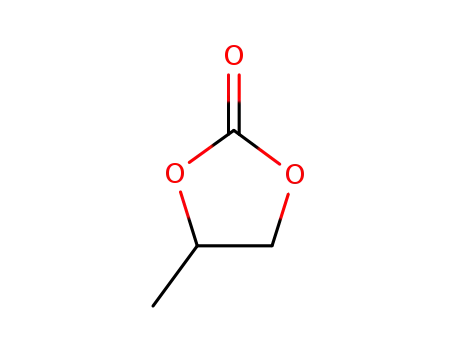
1,2-propylene cyclic carbonate

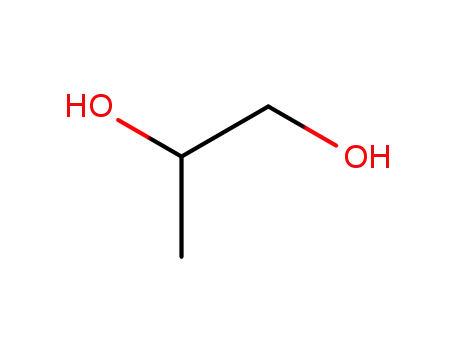
propylene glycol
| Conditions | Yield |
|---|---|
|
tetra-n-butylphosphonium chloride;
at 180 ℃;
for 4h;
under 15001.5 - 37503.8 Torr;
Product distribution / selectivity;
Gas phase;
|
93.4% 0.3% |
|
tetraethylphosphonium bromide;
at 180 ℃;
for 4h;
under 15001.5 - 37503.8 Torr;
Product distribution / selectivity;
Gas phase;
|
92.7% 0.5% |
|
With
1,8-diazabicyclo[5.4.0]undec-7-ene; cellulose;
at 120 ℃;
for 2h;
under 15001.5 Torr;
Reagent/catalyst;
Temperature;
Catalytic behavior;
Autoclave;
Green chemistry;
|
90% |
|
methyl(tributyl)phosphonium iodide;
at 180 ℃;
for 4h;
under 15001.5 - 37503.8 Torr;
Product distribution / selectivity;
Gas phase;
|
75.1% 0.3% |
|
tetra-n-butylphosphonium iodide;
at 180 ℃;
for 4h;
under 15001.5 - 37503.8 Torr;
Product distribution / selectivity;
Gas phase;
|
72% 0.4% |
|
tribenzyl-methyl-phosphonium; bromide;
at 180 ℃;
for 4h;
under 15001.5 - 37503.8 Torr;
Product distribution / selectivity;
Gas phase;
|
70.3% 0.2% |
|
tetrabutyl phosphonium bromide;
at 180 ℃;
for 4h;
under 15001.5 - 37503.8 Torr;
Product distribution / selectivity;
Gas phase;
|
64.8% 0.3% |
|
With
C20H24ClCrN4(2+)*2Cl(1-); tetra-(n-butyl)ammonium iodide;
at 100 ℃;
for 0.5h;
under 127513 Torr;
Catalytic behavior;
Supercritical conditions;
Autoclave;
|
60 %Chromat. |
|
With
rhodium(III) chloride; hydrogen;
In
dimethyl sulfoxide;
at 140 ℃;
for 16.5h;
under 37503.8 Torr;
Reagent/catalyst;
Kinetics;
Autoclave;
|
|
|
With
bis(imidazolate-2-carboxyaldehyde)zinc(II);
In
neat (no solvent);
at 120 ℃;
for 8h;
under 9000.9 Torr;
Pressure;
Temperature;
Catalytic behavior;
Autoclave;
|
81 %Chromat. |
|
With
zinc(II) iodide;
at 140 ℃;
for 6h;
under 15001.5 Torr;
Reagent/catalyst;
Temperature;
Overall yield = 89.5 %;
Catalytic behavior;
Autoclave;
|
|
|
With
water;
In
acetonitrile;
at 40 ℃;
for 1h;
under 3750.38 Torr;
Pressure;
Reagent/catalyst;
Solvent;
Temperature;
Autoclave;
|
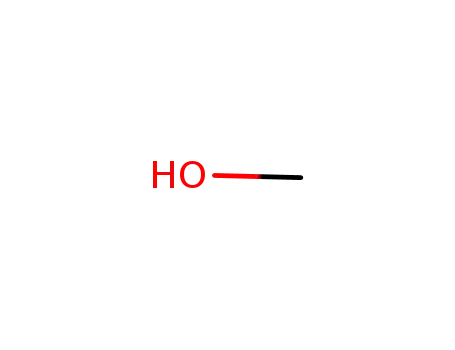
methanol


carbon dioxide


methyloxirane


1,2-propylene cyclic carbonate


propylene glycol

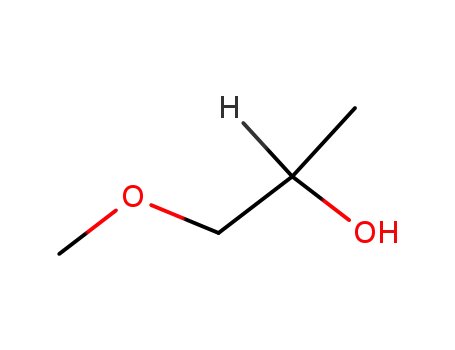
1-methoxy-2-propanol

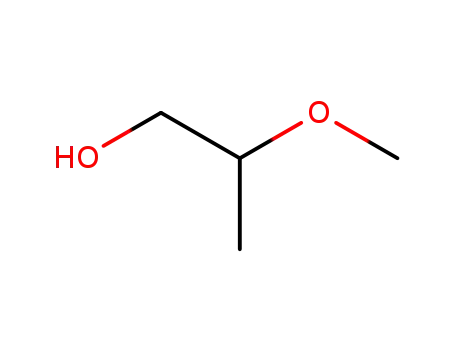
2-methoxypropanol

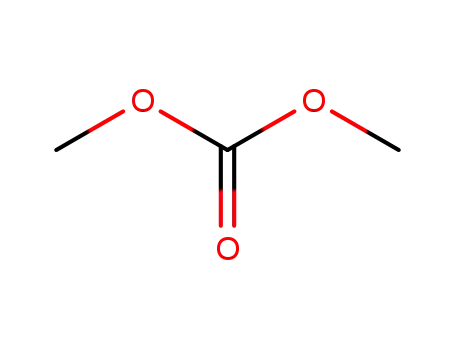
carbonic acid dimethyl ester
| Conditions | Yield |
|---|---|
|
With
6,7,9,10,12,13,20,21-octahydrodibenzo[b,h][1,4,7,10,13,16]hexaoxacyclooctadecine; potassium chloride;
at 130 ℃;
for 8h;
under 15001.5 Torr;
Reagent/catalyst;
Autoclave;
High pressure;
|
13.2% 69.2% 12.5% |
|
With
6,7,9,10,12,13,20,21-octahydrodibenzo[b,h][1,4,7,10,13,16]hexaoxacyclooctadecine; potassium bromide;
at 130 ℃;
for 8h;
under 15001.5 Torr;
Reagent/catalyst;
Autoclave;
High pressure;
|
12.5% 62.9% 14.4% |
|
With
potassium bromide;
at 130 ℃;
for 8h;
under 15001.5 Torr;
Autoclave;
High pressure;
|
5.8% 44.1% 6.7% |
|
With
6,7,9,10,12,13,20,21-octahydrodibenzo[b,h][1,4,7,10,13,16]hexaoxacyclooctadecine; potassium chloride;
at 130 ℃;
for 8h;
under 15001.5 Torr;
Autoclave;
High pressure;
|
23% 41.7% 29.8% |
|
With
potassium carbonate;
at 140 ℃;
for 6h;
Supercritical conditions;
Green chemistry;
|
33.8% 20.3% 36.2% |
|
With
potassium carbonate;
at 160 ℃;
for 6h;
Supercritical conditions;
Green chemistry;
|
36.4% 19.9% 33.9% |
|
With
potassium chloride;
at 130 ℃;
for 8h;
under 15001.5 Torr;
Autoclave;
High pressure;
|
7.8% 35.9% 7.8% |
|
With
Zn4O(1,4-benzenedicarboxylate)3; potassium carbonate; potassium iodide;
at 130 ℃;
for 4h;
under 30003 Torr;
Autoclave;
|
70 %Chromat. 15.3 %Chromat. 17.3 %Chromat. |

propylene glycol
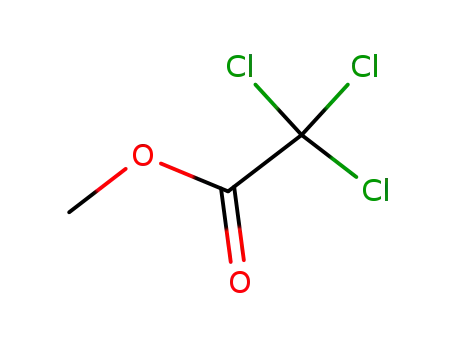
Methyl trichloroacetate
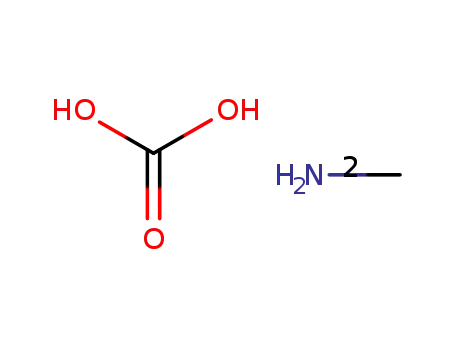
methylammonium carbonate
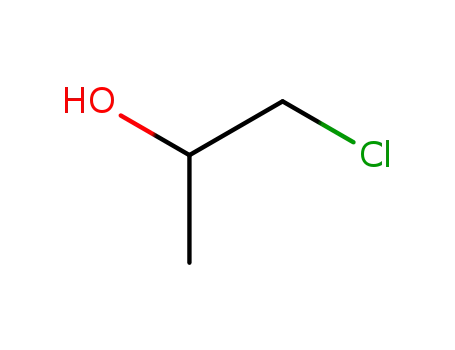
1-Chloro-2-propanol

2-bromo-9H-fluorene
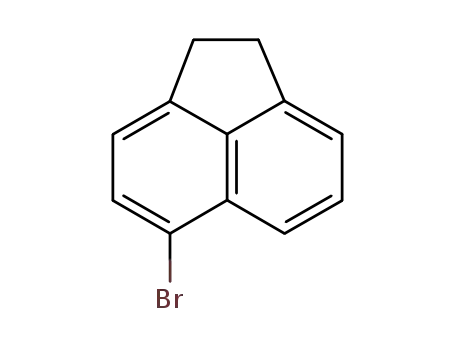
5-bromoacenaphthene
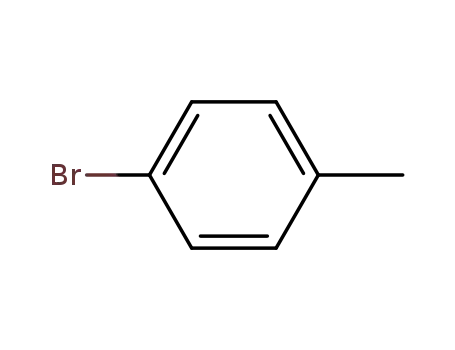
para-bromotoluene
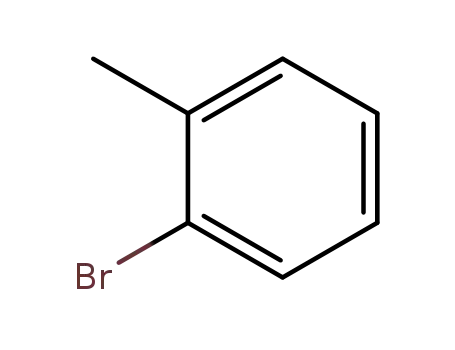
2-methylphenyl bromide