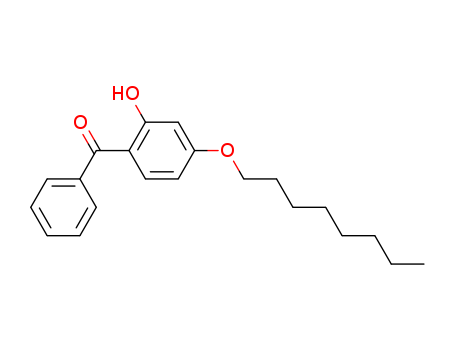
- Product Name
- CasNo
- MF
- MW
- Content
- Appearance
- Packing
- Apply
- UV Absorber531
- 1843-05-6
- C21H26O3
- 326.436
- Light Yellow Crystal
Your Location:Home > Products > UV absorber > UV Absorber531

|
Features/benefits |
UV Absorber 531 is particularly suitable for thick films, typically > 100 μm and thick sections. The low vapor pressure of UV Absorber 531 prevents losses during processing. Low migration rates reduce the risk of blooming. |
|
Analytic Laboratory Methods |
Migration of photo stabilizers from plastics was studied in model elution expt where the plastics were placed at different temp 20 and 45 degree in different solvents, e.g. H2O, acetic acid,? sunflower oil, heptane, and the amt of photostabilizer eluted was determined by spectrophotometry and by gas chromatography. The elution of 2-hydroxy-4-n-octyloxybenzophenone depended on the type of plastic. Elution was low in polystyrene and high in polyolefins, polyethylene. Food contamination from these plastic additives is discussed. |
|
Handling & Safety |
In accordance with good industrial practice, handle with care and avoid unnecessary personal contact. Avoid continuous or repetitive breathing of dust. Use only with adequate ventilation. Protect skin. Avoid dust formation and ignition sources. For more detailed information please refer to the material safety data sheet. |
|
Preparation |
Preparation by reaction of n-octyl chloride with 2,4-di-hydroxybenzophenone, ? in the presence of a mixture of sodium carbonate, triethylamine and potassium iodide in refluxing butanol for 15 h (90%); ? in the presence of potassium carbonate in cyclohexanone at 145° for 5 h (66%); ? in the presence of potassium hydroxide and antimony triiodide in diethylene glycol at 150° for 1 h (93%); ? in the presence of sodium bicarbonate and potassium iodide in 1-methylpyrrolidone for 2 h at 150° (96%). |
|
Flammability and Explosibility |
Notclassified |
|
Characterization |
UV Absorber 531 is an ultraviolet light absorber (UVA) of the benzophenone class, imparting good light stability when used in combination with a hindered amine light stabilizer (HALS) of the Chimassorb, Tinuvin? or Uvinul? range. It shows good compatibility with polyolefins and plasticized PVC. |
|
Applications |
The main application of UV Absorber 531 is in combination with a HALS the light stabilization of low density and linear low density polyethylene as well as ethylene-vinyl acetate copolymers for agricultural films. It can be used as well as a UV barrier to protect the contents of packages for both industrial and consumer applications. Also, in combination with HALS, UV Absorber 531 can be used in high density polyethylene molded articles, e. g. in crates. UV Absorber 531 also protects a number of other polymers against degradation caused by light exposure such as plasticized PVC and rubbers. UV Absorber 531 can be used in combination with antioxidants, phosphites and other light stabilizers. |
|
General Description |
2-Hydroxy-4-(octyloxy)benzophenone (HOBP) is a UV based absorber that has 2-hydroxybenzophenone as a functional group. It can be used to enhance the light fastness of polymers using UV absorbers with sulfur and phosphorus compounds. |
InChI:InChI=1/C21H26O3/c1-2-3-4-5-6-10-15-24-18-13-14-19(20(22)16-18)21(23)17-11-8-7-9-12-17/h7-9,11-14,16,22H,2-6,10,15H2,1H3
The invention discloses a novel process ...
The invention discloses a process for sy...
Suggested is a cosmetic compositions com...
Suggested is a composition comprising (a...

2.4-dihydroxybenzophenone

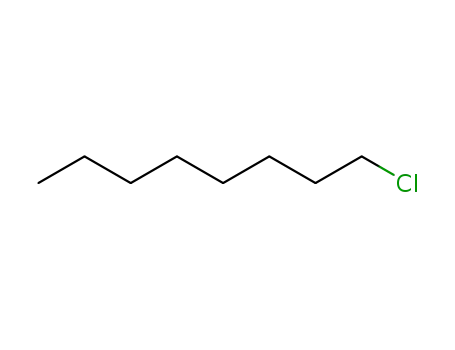
n-chlorooctane

Cyasorb UV531
| Conditions | Yield |
|---|---|
|
With
sodium carbonate; potassium carbonate;
at 98 - 120 ℃;
Temperature;
|
95.3% |
|
With
sodium carbonate;
In
N,N-dimethyl-formamide;
at 70 ℃;
for 8h;
Temperature;
Reagent/catalyst;
Solvent;
|
95% |

Cyasorb UV531
| Conditions | Yield |
|---|---|
|
2,4-Dihydroxybenzophenon, Octylchlorid, KOH, SbI3;
|
|
|
Resorcin, Benzotrichlorid, n-Octylchlorid;
|
|
|
Resorcin, Benzotrichlorid, n-Octylbromid;
|
|
|
Resorcin, Benzotrichlorid, n-Octyl-p-toluolsulfonat;
|
|
|
Resorcin, Benzoylchlorid, n-Octylbromid;
|
|
|
Keton 1, n-Octylbromid
|
|
|
entspr. 2,4-Dihydroxyverb., n-Octylchlorid-K2CO3-Kat.;
|
|
|
Benzophenon-ammoniumiodid (II), n-Octylchlorid;
|
|
|
2,4-Dihydroxybenzophenon (II), Octylbromid;
|
|
|
/BRN= 144597/; SeO2, (Dioxan);
|
|
|
/BRN= 311566/ (I) + n-Octyl-halogenid in alkal. Lsg.;
|
|
|
1-Brom- bzw. 1-Chlor-octan, 2,4-Dihydroxybenzophenon in Ggw. v. K-hydroxid und oberflaechenaktiven Substanzen;
|
|
|
/BRN= 311566/, C8H17Cl, Aethylenglycol-monoaethylaether (Na2CO3 u. KI enthaltend);
|
|
|
entspr.Hydroxybenzophenon, entspr.Phenylsulfonat;
|
|
|
2,4-Dihydroxy-benzophenon, wss.-ethanol. NaOH, Octylbromid;
|
|
|
Resorcindioctylether, PhCOCl
|
|
|
entspr. Phenol, n-C8H17Cl;
|
|
|
entspr. Phenol, n-C8H17Br;
|
|
|
entspr. Phenol, n-C8H17O-Tos;
|
|
|
2,4-Dihydro-benzophenon, Octylbromid;
|
|
|
2,4-Dihydro-benzophenon, n-Octyl-p-toluolsulfonat;
|
|
|
2,4-Dihydro-benzophenon, n-Octylbenzolsulfonat;
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|

2.4-dihydroxybenzophenone

n-chlorooctane
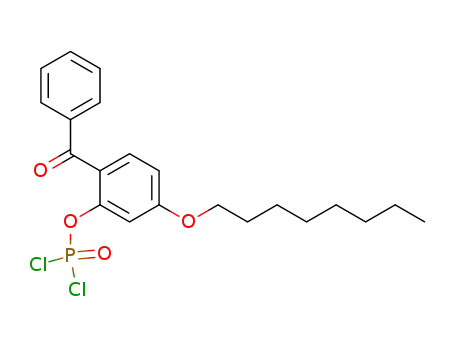
C21H25Cl2O4P
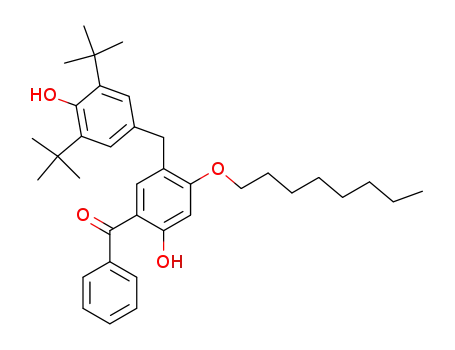
5-(3,5-di-tert-butyl-4-hydroxybenzyl)-2-hydroxy-4-octyloxybenzophenone
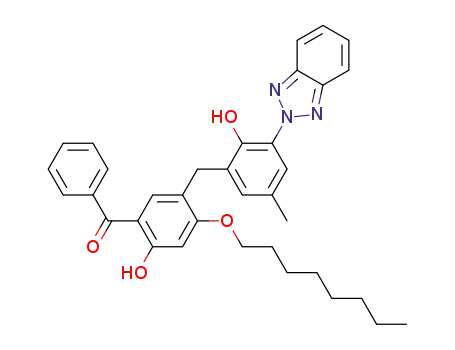
[2,4'-Dihydroxy-3-(2H-benzotriazol-2-yl)-5-methyl-2'-n-octoxy-5'-benzoyl]diphenylmethane
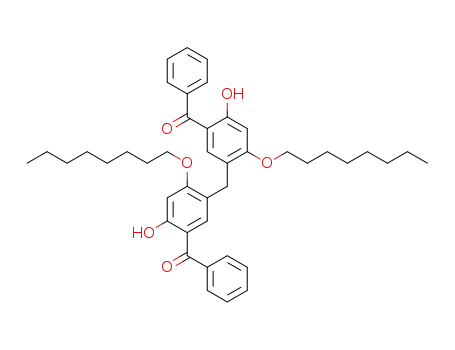
5,5'-methylenebis(2-hydroxy-4-n-octyloxybenzophenone)