- Product Name
- CasNo
- MF
- MW
- Content
- Appearance
- Packing
- Apply
- UV Absorber 326
- 3896-11-5
- C17H18ClN3O
- 315.802
- powder
Your Location:Home > Products > UV absorber > UV Absorber 326
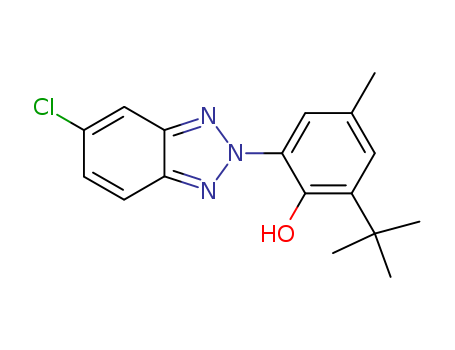

|
Features/Benefits |
UV Absorber 326 has a wide range of indirect food approvals in polyolefins. It has a low volatility at high temperatures and high resistance to thermal degradation and can therefore be used without significant loss or decomposition in the polyolefin compounding and molding processes. In the use for the UV protection of polyester resins, TINUVIN 326 does not form colored complexes with the metallic salts used for the curing process of these resins. |
|
Handling & Safety |
In accordance with good industrial practice, handle with care and avoid unnecessary personal contact. Avoid continuous or repetitive breathing of dust. Use only with adequate ventilation. Prevent contamination of the environment. Avoid dust formation and ignition sources. For more detailed information please refer to the material safety data sheet. |
|
Manufacturing Process |
To a 2000 ml 3-necked, round-bottomed flask equipped with an agitator, reflux condenser, nitrogen inlet and thermometer were charged 140.5 g of 2'- hydroxy-3'-t-butyl-5'-methyl-5-chloro-2-nitroazobenzene, 119 g of isopropanol and 80 g of Amsco mineral spirits. A stream of nitrogen was introduced over the surface of the contents of the flask and the nitrogen atmosphere was then maintained throughout the remainder of the reduction process. 13.7 g of 50% aqueous sodium hydroxide solution and 222 g of water were added and the temperature of the contents of the flask were adjusted to 55°C. The ratio of the moles of alkali to moles of o-nitroazobenzene intermediate used was is 0.848/1. 104 g of zinc dust was added in 5 portions over a 2 hours period with the temperature of the flask being held at 55-60°C with some slight external cooling. The total concentration of iron impurities from all reactants less than 150 ppm based on zinc used. After all the zinc was added, the temperature was raised to 60°C and held at this temperature until a spot test indicated no more o-nitroazobenzene intermediate was present. The temperature was then raised to 65°C and held there for 4 to 5 hours or until TLC analysis indicated that no more of the N-oxy-intermediate was present. 62.6 g of anhydrous sodium sulfate and 35.6 g of water were then added, the batch was heated to 70°C and stirred for 15 min. The material was then allowed to stand and separate into three liquid phases plus unreacted zinc dust. The top two layers containing the desired product were transferred to another flask. The remaining aqueous zinc slurry was washed at 65-70°C with three successive 16 g portions of Amsco mineral spirits: isopropanol 50:50. The combined product layers and wash liquids were then washed once at 70°C with an aqueous hydrochloric acid solution made from 130 g of water and 40 g of 32% hydrochloric acid to remove cleavage amine by-products. A second and third wash followed at 70°C with aqueous hydrochloric acid solutions made each from 65 g of water and 20 g of 32% hydrochloric acid. The last wash was essentially colorless. 14 g of 32% hydrochloric acid and 220 g of isopropanol were added to the solution of the product. The batch was allowed to crystallize slowly. The solid product form was filtered and washed with isopropanol at 0°C to give 110 g of 5-chloro-2-(2-hydroxy-3-t-butyl-5- methylphenyl)-2H-benzotriazole with a melting point of 140-141°C. |
|
Therapeutic Function |
Sunscreen agent |
|
Flammability and Explosibility |
Notclassified |
|
Characterization |
UV Absorber 326 is a UV absorber of the hydroxyphenylbenzotriazole class, which imparts outstanding light stability to plastics and other organic substrates. |
|
Applications |
UV Absorber 326 is especially suited for polyolefins and cold cured polyesters. |
|
General Description |
2-tert-Butyl-6-(5-chloro-2H-benzotriazol-2-yl)-4-methylphenol (UV-326) is a benzotriazole based UV absorber that can be used in the photostabilization of industrial materials. It can absorb the harmful UV radiation for the wavelength of about 290 nm. |
InChI:InChI=1/C17H18ClN3O/c1-10-7-12(17(2,3)4)16(22)15(8-10)21-19-13-6-5-11(18)9-14(13)20-21/h5-9,22H,1-4H3
The invention relates to a preparation m...
The invention relates to a method to pre...
The invention relates to a preparation m...
The invention discloses a preparation me...
C17H18ClN3O2

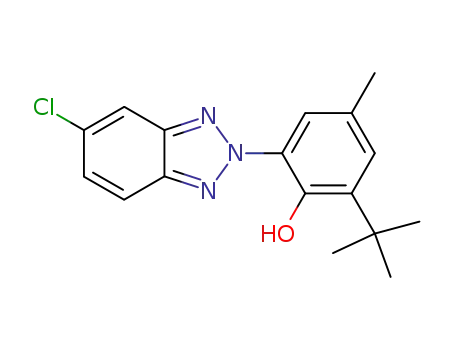
2-(3-tert-butyl-2-hydroxy-5-methylphenyl)-2H-5-chlorobenzotriazole
| Conditions | Yield |
|---|---|
|
With
platinum on activated charcoal; hydrogen;
at 50 - 75 ℃;
under 3750.38 - 6000.6 Torr;
Autoclave;
|
92.1% |
C17H20ClN3O2


2-(3-tert-butyl-2-hydroxy-5-methylphenyl)-2H-5-chlorobenzotriazole
| Conditions | Yield |
|---|---|
|
With
sodium phosphite; hydrogen; sodium hydroxide;
at 80 ℃;
for 8h;
under 11251.1 Torr;
Reagent/catalyst;
Pressure;
Temperature;
Autoclave;
|
94.3% |
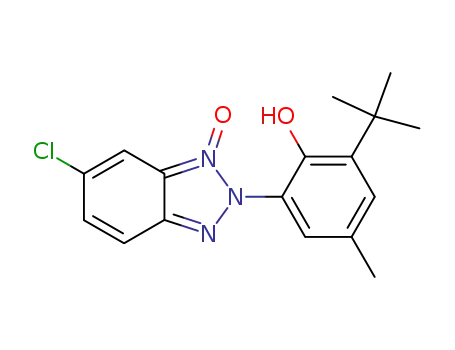
2-tert-Butyl-6-(6-chloro-1-oxy-benzotriazol-2-yl)-4-methyl-phenol
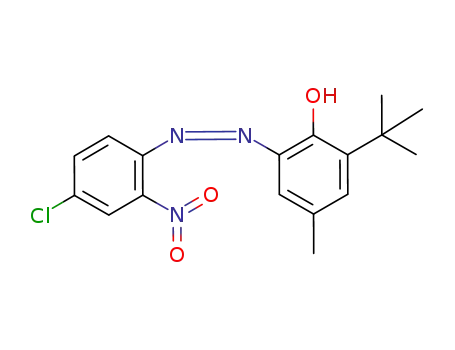
2-<(4-Chloro-2-nitrophenyl)azo>-6-(1,1-dimethylethyl)-4-methylphenol
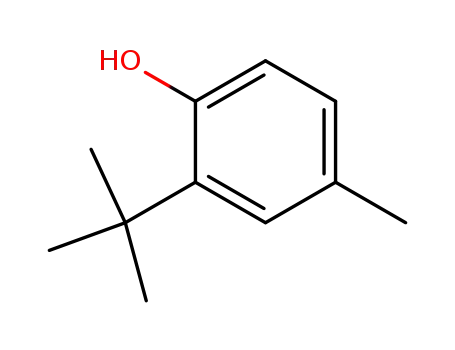
2-tert-Butyl-4-methylphenol
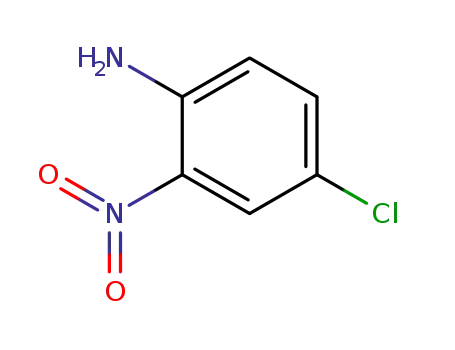
4-Chloro-2-nitroaniline
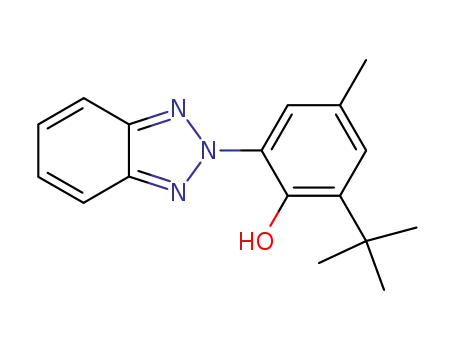
2-(2-hydroxy-3-tert-butyl-5-methylphenyl)-2H-benzotriazole
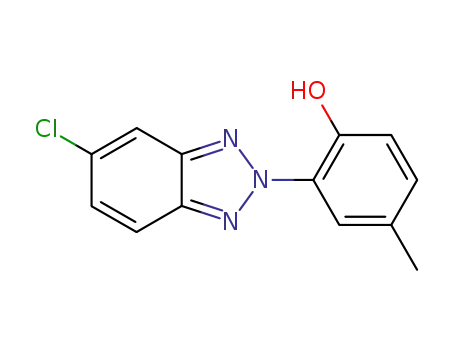
5-chloro-2-(2-hydroxy-5-methylphenyl)-2H-benzotriazole
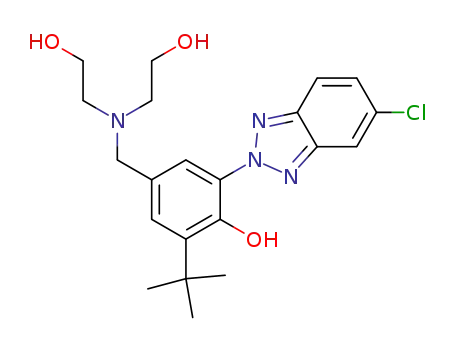
2-(2'-Hydroxy-3'-tert-butyl-5'-(bis(2-hydroxyethyl)aminomethyl)phenyl)-5-chlorobenzotriazole
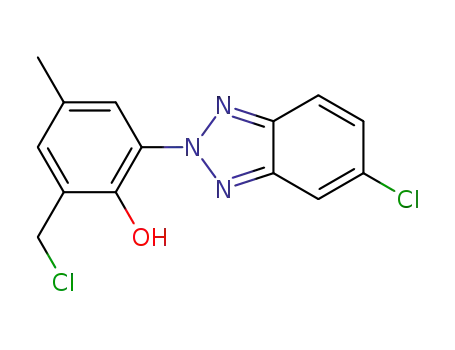
2-(5-chloro-2H-benzotriazol-2-yl)-6-chloromethyl-4-methylphenol