- Product Name
- CasNo
- MF
- MW
- Content
- Appearance
- Packing
- Apply
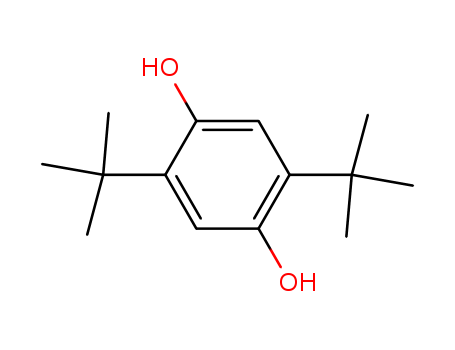
- 2,5-Di-tert-butylhydroquinone (DBTHQ)
- 88-58-4
- C14H22O2
- 222.327
- White or light yellow crystals
Your Location:Home > Products > Antioxidants > 2,5-Di-tert-butylhydroquinone (DBTHQ)

|
Biological Activity |
A selective inhibitor of endoplasmic reticulum Ca 2+ -ATPase. |
|
Biochem/physiol Actions |
2,5-Di-tert-butylhydroquinone specifically inhibits the sarcoplasmic reticulum (SR) Ca2+ uptake in the rat ventricle. |
|
Purification Methods |
Crystallise the hydroquinone from *C6H6 or AcOH. [Beilstein 6 III 4741.] |
|
references |
[1]. hasséssian h, vaca l, kunze dl. blockade of the inward rectifier potassium current by the ca(2+)-atpase inhibitor 2',5'-di(tert-butyl)-1,4-benzohydroquinone (bhq). br j pharmacol, 1994, 112(4): 1118-1122.[2]. fusi f, gorelli b, valoti m, et al. effects of 2,5-di-t-butyl-1,4-benzohydroquinone (bhq) on rat aorta smooth muscle. eur j pharmacol, 1998, 346(2-3): 237-243.[3]. fusi f, saponara s, gagov h, et al. 2,5-di-t-butyl-1,4-benzohydroquinone (bhq) inhibits vascular l-type ca(2+) channel via superoxide anion generation. br j pharmacol, 2001, 133(7): 988-996.[4]. jan cr, ho cm, wu sn, et al. mechanism of rise and decay of 2,5-di-tert-butylhydroquinone-induced ca2+ signals in madin darby canine kidney cells. eur j pharmacol, 1999, 365(1): 111-117. |
|
Definition |
ChEBI: A member of the class of hydroquinones that is benzene-1,4-diol substituted by tert-butyl groups at position 2 and 5. |
|
General Description |
Mobilizes Ca2+ specifically from the Ins(1,4,5)P3-sensitive Ca2+ stores by inhibiting microsomal and sarcoplasmic reticulum Ca2+-ATPase activity. Does not affect mitochondrial Ca2+ fluxes or plasma membrane Ca2+/Mg2+ ATPase activity. Useful for the study of endomembrane Ca2+ stores and plasma membrane Ca2+ permeability pathways. |
InChI:InChI=1/C14H22O2/c1-13(2,3)9-7-12(16)10(8-11(9)15)14(4,5)6/h7-8,15-16H,1-6H3
The invention discloses a preparation me...
A PROCESS FOR PREPARATION OF TERTIARY BU...
Pro-aromatic and volatile 1-methyl-1,4-c...
In the interest of investigating new hyd...
acid blue 5

5-methyl-2-hydroxyacetophenone

4-hydroxybutanoic acid

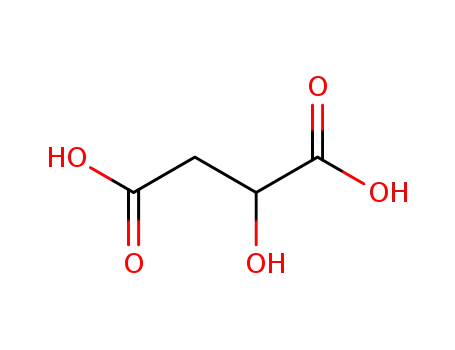
malic acid

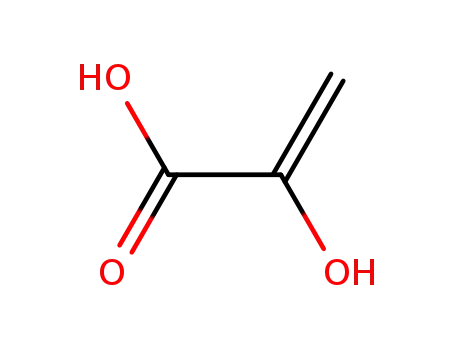
2-hydroxyacrylic acid

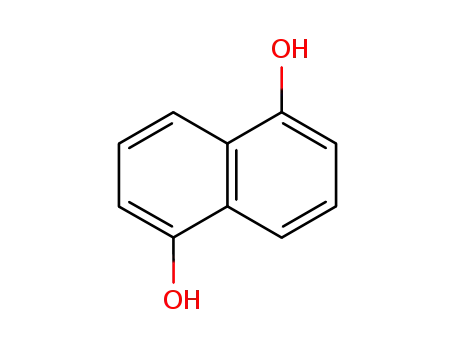
1,5-dihydroxynaphthalene

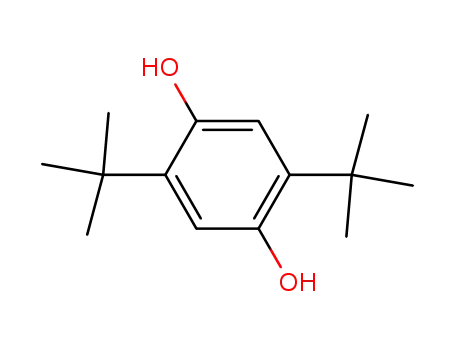
2,5-bis(1,1-dimethylethyl)-1,4-benzenediol

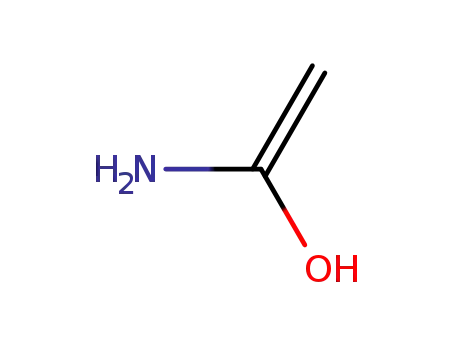
1-aminoethenol
| Conditions | Yield |
|---|---|
|
With
dihydrogen peroxide;
pH=3;
Reagent/catalyst;
Concentration;
pH-value;
Kinetics;
|
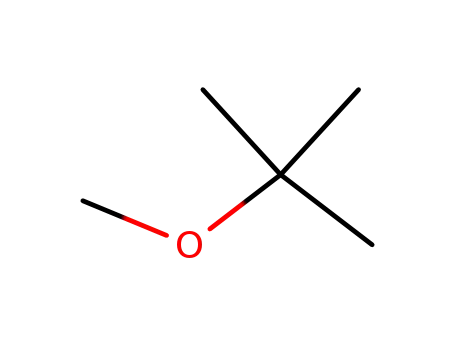
tert-butyl methyl ether

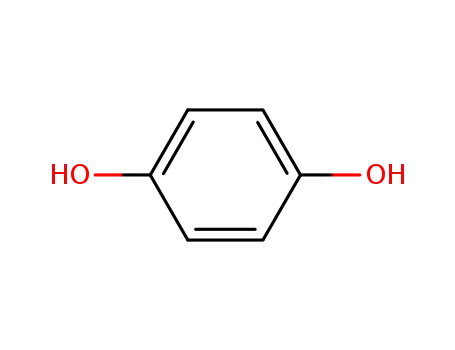
hydroquinone

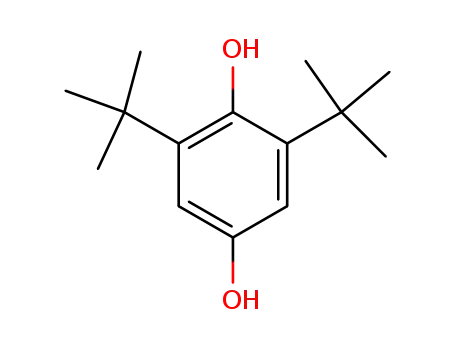
2,6-di-tert-butyl-4-hydroxyphenol


2,5-bis(1,1-dimethylethyl)-1,4-benzenediol

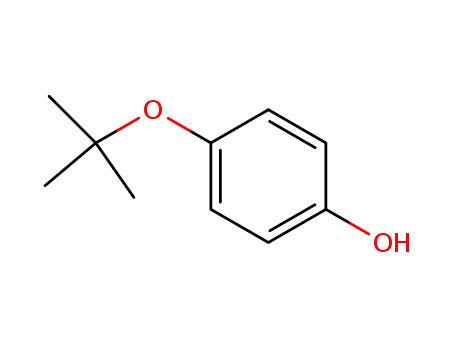
4-(tert-butoxy)phenol

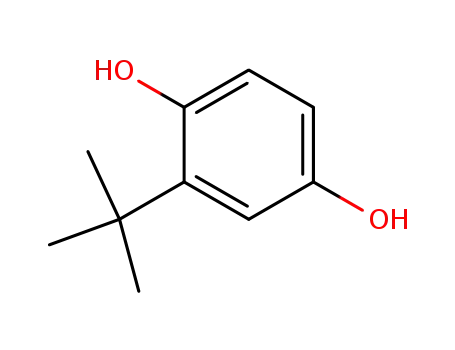
tert-butylhydroquinone
| Conditions | Yield |
|---|---|
|
With
3-(4-sulfobutylamino)propylsilanized MCM-41;
In
nitrobenzene;
at 150 ℃;
for 0.133333h;
Concentration;
chemoselective reaction;
Catalytic behavior;
Microwave irradiation;
Green chemistry;
|
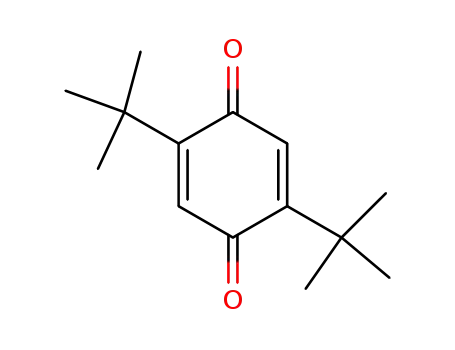
2,5-di-tert-butyl-p-benzoquinone
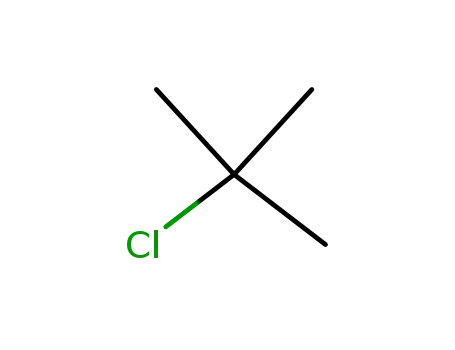
tertiary butyl chloride

hydroquinone
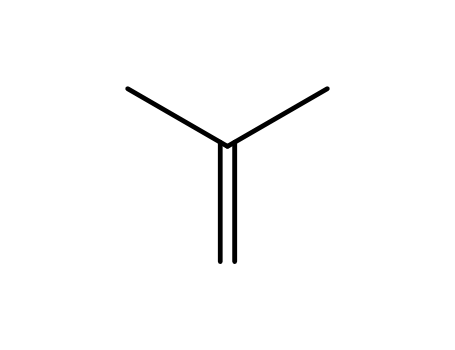
isobutene

2,5-di-tert-butyl-p-benzoquinone
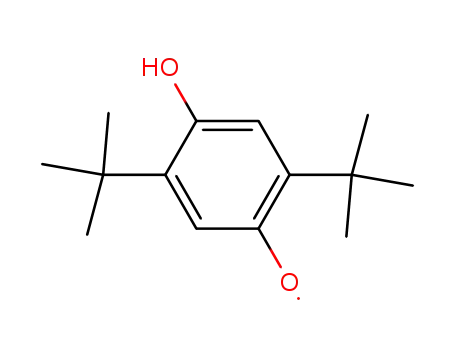
2,5-Di-tert-butyl-4-hydroxy-phenoxyl
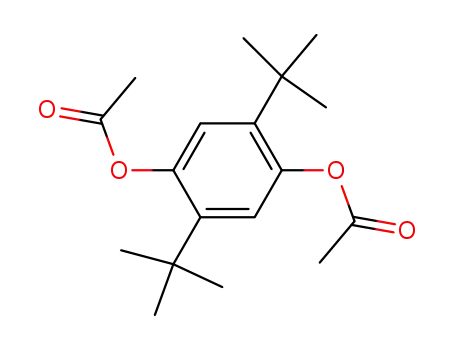
1,4-diacetoxy-2,5-di-tert-butyl-benzene
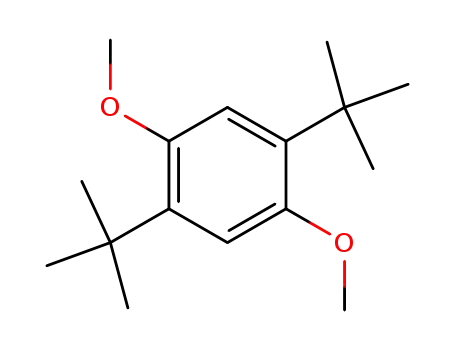
1,4-di-tert-butyl-2,5-dimethoxybenzene