- Product Name
- CasNo
- MF
- MW
- Content
- Appearance
- Packing
- Apply
- 4-Methylmorpholine N-oxide
- 7529-22-8
- C5H11NO2
- 117.148
- Clear colorless to yellow solution
Your Location:Home > Products > Catalysts and additives > 4-Methylmorpholine N-oxide


|
Flammability and Explosibility |
Flammable |
|
Purification Methods |
When the oxide is dried for 2-3hours at high vacuum, it dehydrates. Add MeOH to the oxide and distil off the solvent under vacuum until the temperature is ca 95o. Then add Me2CO at reflux and cool to 20o. The crystals are filtered off, washed with Me2CO and dried. The degree of hydration may vary and may be important for the desired reactions. [van Rheenan et al. Tetrahedron Lett 1973 1076, Schneider & Hanze US Pat 2 769 823; see also Sharpless et al. Tetrahedron Lett 2503 1976.] |
|
General Description |
4-Methylmorpholine?N-oxide is an organic compound used as a co-oxidant along with OsO4 and ruthenates in organic synthesis. In recent studies, it has been used as a catalyst in silylcyanation of aldehydes and ketones. Lyocell, a regenerated cellulose fiber, can be prepared using 4-methylmorpholine?N-oxide in an eco-friendly manner. |
InChI:InChI=1/C5H11NO2/c1-6(7)2-4-8-5-3-6/h2-5H2,1H3
An efficient synthesis of N-(trideuterom...
A method for producing an amine oxide by...
The invention provides a preparation met...
The invention belongs to the technical f...
A series of flavinium salts, 5-ethylisoa...
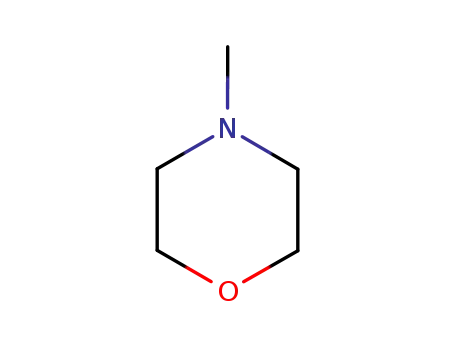
4-methyl-morpholine

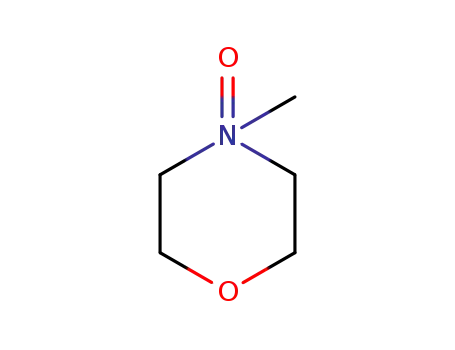
4-methylmorpholine N-oxide
| Conditions | Yield |
|---|---|
|
With
4-hydroperoxy-5-ethyl-3-methyllumiflavine;
In
tert-butyl alcohol;
at 30 ℃;
Rate constant;
|
100% |
|
With
carbon dioxide; phosphoric acid; dihydrogen peroxide;
for 0.133333h;
under 7500.75 Torr;
Reagent/catalyst;
Pressure;
Time;
Autoclave;
|
100% |
|
With
dihydrogen peroxide; benzonitrile;
Mg-Al-O-t-Bu HT (Catalyst B);
In
methanol; water;
at 65 ℃;
for 0.5h;
Product distribution / selectivity;
|
98% |
|
With
oxidized flavin; oxygen; hydrazine hydrate;
In
2,2,2-trifluoroethanol;
at 60 ℃;
for 2h;
under 760.051 Torr;
|
97% |
|
With
dihydrogen peroxide; benzonitrile;
Mg-Al-O-t-Bu HT (Catalyst C);
In
methanol; water;
at 65 ℃;
for 0.5h;
Product distribution / selectivity;
|
97% |
|
With
dihydrogen peroxide; benzonitrile;
Mg-Al-O-t-Bu HT (Catalyst D);
In
methanol; water;
at 65 ℃;
for 0.5h;
Product distribution / selectivity;
|
97% |
|
With
dihydrogen peroxide; benzonitrile;
Mg-Al-O-t-Bu HT (Catalyst E);
In
methanol; water;
at 65 ℃;
for 0.5h;
Product distribution / selectivity;
|
97% |
|
With
dihydrogen peroxide;
layered double hydroxide WO4(2-);
at 20 ℃;
for 1h;
|
96% |
|
With
dihydrogen peroxide; benzonitrile;
Mg-Al-O-t-Bu HT (Catalyst A);
In
methanol; water;
at 65 ℃;
for 0.5h;
Product distribution / selectivity;
|
96% |
|
With
2,2,2-Trifluoroacetophenone; dihydrogen peroxide; acetonitrile;
In
tert-butyl alcohol;
at 20 ℃;
for 18h;
chemoselective reaction;
Green chemistry;
|
95% |
|
With
dihydrogen peroxide; benzonitrile;
Mg-Al-O-t-Bu HT (Catalyst B);
In
water;
at 65 ℃;
for 0.5h;
Product distribution / selectivity;
|
92% |
|
With
2-hydroxyperoxyhexafluoro-2-propanol;
In
dichloromethane;
at 0 ℃;
for 0.166667h;
|
90% |
|
With
oxaziridinium tetrafluoroborate derived from N-methyl-1,2,3,4-tetrahydroisoquinoline;
In
dichloromethane;
at -18 ℃;
|
90% |
|
With
dihydrogen peroxide; sodium hydrogencarbonate; trichloroacetonitrile;
In
tetrahydrofuran; water;
at 0 - 25 ℃;
for 4h;
|
89% |
|
With
dihydrogen peroxide;
In
water;
at 20 ℃;
for 3h;
|
83% |
|
With
1,3-dimethyl-5-ethyl-5,10-dihydroalloxazine; dihydrogen peroxide;
In
methanol; water;
for 2h;
Ambient temperature;
|
75% |
|
With
dihydrogen peroxide; benzonitrile;
potassium tert-butylate;
In
methanol; water;
at 65 ℃;
for 1.1h;
Product distribution / selectivity;
|
75% |
|
With
iron(III) oxide; oxygen; isovaleraldehyde;
In
1,2-dichloro-ethane;
at 40 ℃;
for 3h;
|
70% |
|
With
oxygen;
ruthenium trichloride;
In
1,2-dichloro-ethane;
at 20 ℃;
for 12h;
under 760 Torr;
|
70% |
|
With
dihydrogen peroxide;
In
water; acetonitrile;
at 80 ℃;
for 4h;
Green chemistry;
|
70% |
|
With
dihydrogen peroxide;
at 70 - 75 ℃;
for 4h;
|
67% |
|
With
oxygen;
In
water;
at 115 ℃;
for 72h;
under 53254.2 Torr;
|
62% |
|
With
dihydrogen peroxide;
In
d(4)-methanol; water;
rate enhancement in the presence of 1,3-dimethyl-5-ethyl-5,10-dihydroalloxazine;
|
|
|
With
3-bromo-4,5-dihydro-5-hydroperoxy-4,4-dimethyl-3,5-diphenyl-3H-pyrazole;
In
chloroform-d1;
at 34 ℃;
further reagents: MCPB;
|
95 % Spectr. |
|
With
dihydrogen peroxide;
In
methanol;
|
|
|
With
3,3-dimethyldioxirane;
In
dichloromethane; acetone;
at 0 ℃;
|
|
|
With
substituted 3-hydroperoxy-1,2-benzisothiazole 1,1-dioxide;
In
chloroform-d1;
at 20 ℃;
for 3h;
|
95 % Spectr. |
|
With
dihydrogen peroxide;
In
water;
at 20 ℃;
|
|
|
With
3-chloro-benzenecarboperoxoic acid;
In
chloroform-d1;
|
|
|
With
3-chloro-benzenecarboperoxoic acid;
In
chloroform-d1;
|
|
|
With
pyridine; 3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
at 0 ℃;
for 2h;
chemoselective reaction;
|
|
|
With
dihydrogen peroxide;
In
water;
at 0 - 30 ℃;
|
|
|
With
dihydrogen peroxide;
In
ethanol;
at 65 - 70 ℃;
for 6h;
|
|
|
With
5-ethyl-1,3,7,8-tetramethylalloxazinium triflate; dihydrogen peroxide;
In
d(4)-methanol; water;
at 25 ℃;
Reagent/catalyst;
|
|
|
With
zinc(II) oxide; dihydrogen peroxide;
In
water;
at 40 ℃;
Temperature;
|
|
|
4-methyl-morpholine;
With
titanium(IV) oxide;
for 0.333333h;
With
dihydrogen peroxide;
at 40 ℃;
Temperature;
|
![4-[6-hydroxy-2,7,8-trimethyl-2-(4,8,12-trimethyl-tridecyl)-chroman-5-ylmethyl]-4-methyl-morpholin-4-ium; iodide](/upload/2024/12/af69e392-6575-424d-9b19-db44ad39f5ad.png)
4-[6-hydroxy-2,7,8-trimethyl-2-(4,8,12-trimethyl-tridecyl)-chroman-5-ylmethyl]-4-methyl-morpholin-4-ium; iodide


4-methylmorpholine N-oxide
| Conditions | Yield |
|---|---|
|
With
sodium percarbonate;
In
hexane; dichloromethane; acetonitrile;
at 50 ℃;
for 1h;
|

4-methyl-morpholine

3-bromo-4,5-dihydro-5-hydroperoxy-4,4-dimethyl-3,5-diphenyl-3H-pyrazole
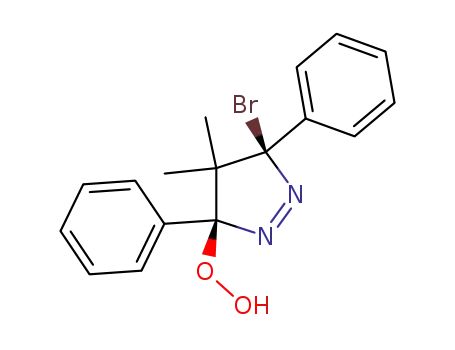
cis-3-bromo-4,5-dihydro-5-hydroperoxy-4,4-dimethyl-3,5-diphenyl-3H-pyrazole
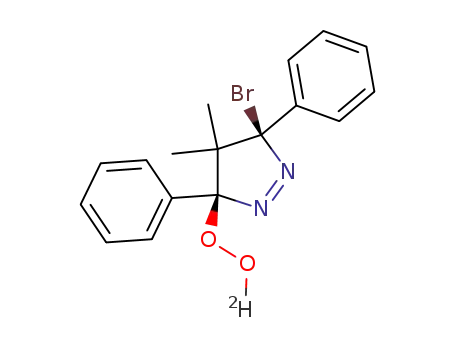
C17H16(2)HBrN2O2
1-morpholino-2-(benzylamino)propane

4-methyl-morpholine

4-hydroxy-3-morpholin-4-ylmethylbenzoic acid methyl ester
morpholine