- Product Name
- CasNo
- MF
- MW
- Content
- Appearance
- Packing
- Apply
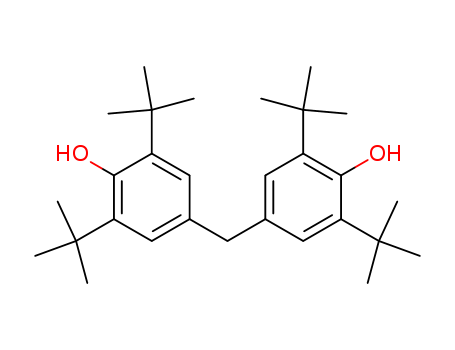
- 4,4'-Methylenebis(2,6-di-tert-butylphenol)
- 118-82-1
- C29H44O2
- 424.667
Your Location:Home > Products > Antioxidants > 4,4'-Methylenebis(2,6-di-tert-butylphenol)

The catalytic air oxidation of p-cresol ...
2-Hydroxymethyl-6-isobornyl-4-methylphen...
Products of thermolysis of 2,6-di-tert-b...
The invention provides a phenolic compou...
The invention provides a phenol derivati...
We present here proton-exchanged montmor...
An iridium-catalyzed hydrogen-borrowing ...
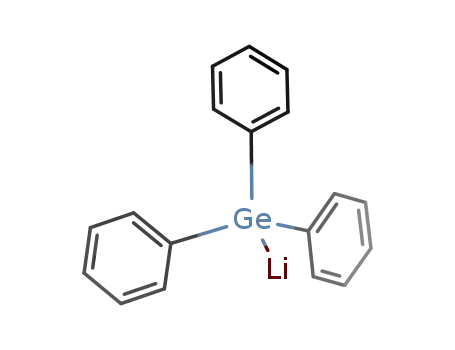
triphenyl germyllithium

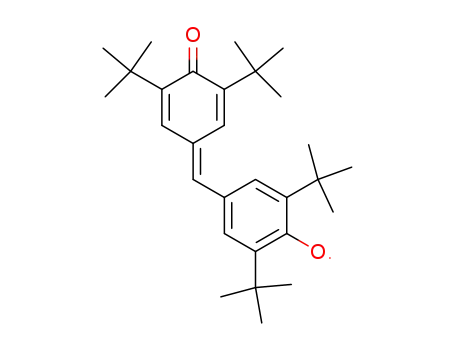
Coppinger's Radikal

(C6H5)3GeCH(C6H2(C4H9)2(OH))2

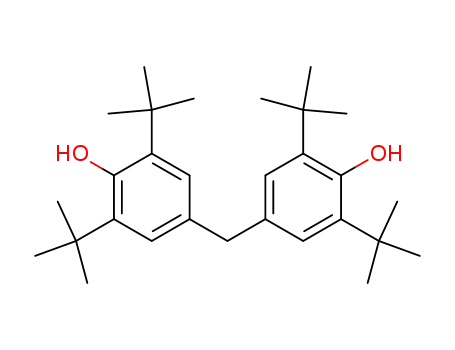
4,4'-Methylenebis(2,6-di-tert-butylphenol)

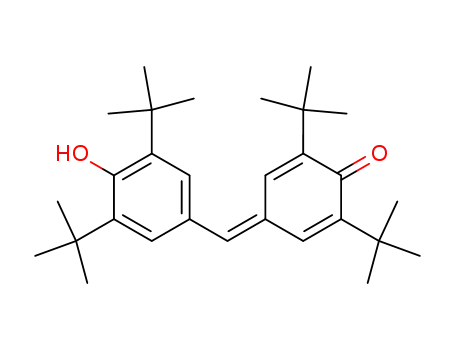
2,6-di-tert-butyl-4-(3,5-di-tert-butyl-4-hydroxy-benzylidene)-cyclohexa-2,5-dienone


hexaphenyldigermane

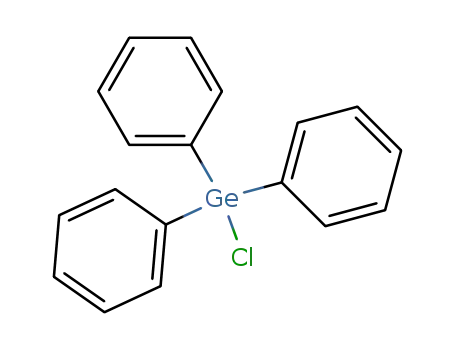
chlorotriphenylgermane
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
tetrahydrofuran;
inert atmosphere; dropwise addn. of org. compd. soln. to soln. of Ge-compd. at 0°C, soln. hydrolysis by dropwise HCl addn.; soln. filtration to obtain Ph3GeGePh3, filtrate extn. (ether), drying (Na2SO4), soln. concn. (reduced pressure); (1)H-NMR spectroscopy;
|
66% 24% 8% 56% 10% |
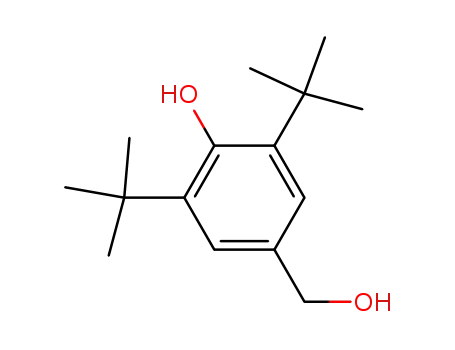
3,5-di-tert-butyl-4-hydroxybenzyl alcohol

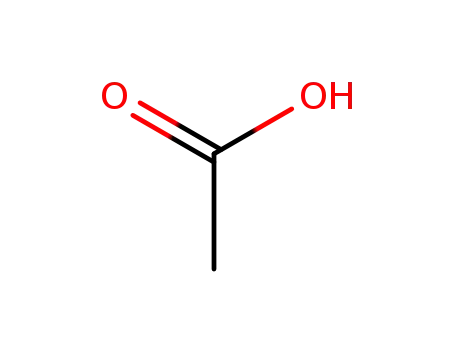
acetic acid

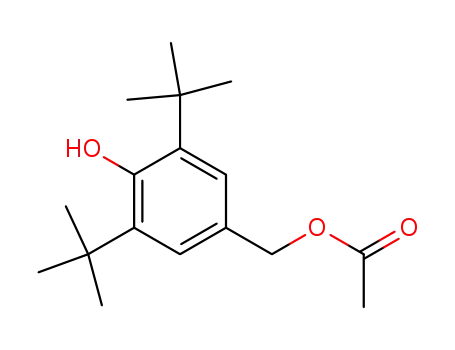
3,5-di-tert-butyl-4-hydroxybenzyl acetate

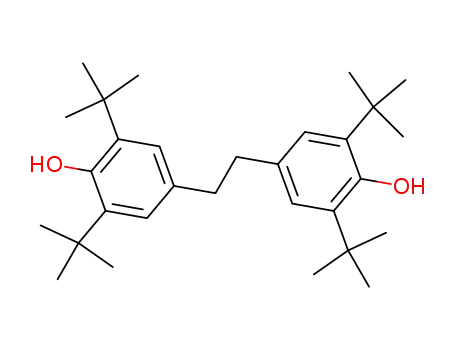
1,2-bis(3,5-di-tert-butyl-4-hydroxyphenyl)ethane


4,4'-Methylenebis(2,6-di-tert-butylphenol)

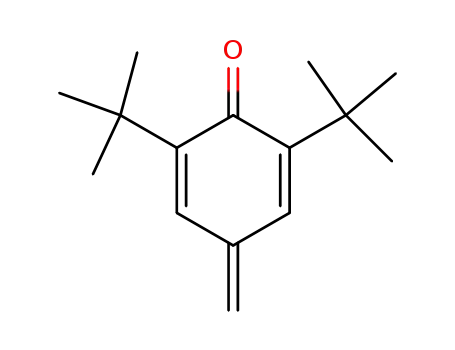
2,6-di-tert-butyl-4-methylene-2,5-cyclohexadien-1-one

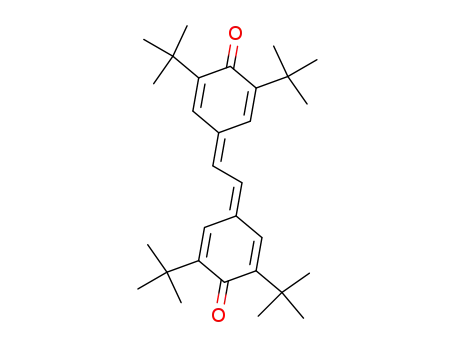
3,3',5,5'-tetra-tert-butyl-4,4'-stilbenequinone

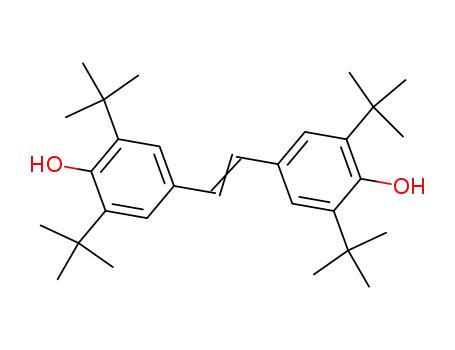
1,2-di(3',5'-di-tert-butyl-4'-hydroxyphenyl)ethene
| Conditions | Yield |
|---|---|
|
With
potassium acetate;
In
water;
at 100 ℃;
for 0.5h;
|

formaldehyd
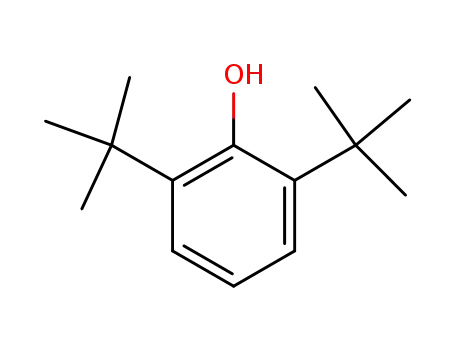
2,6-di-tert-butylphenol
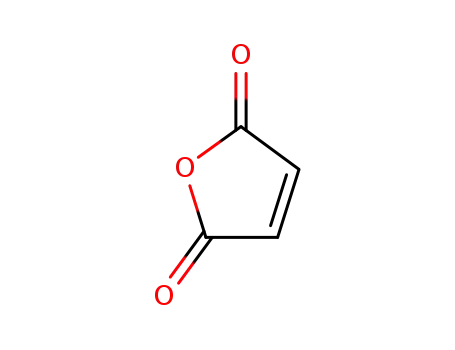
maleic anhydride
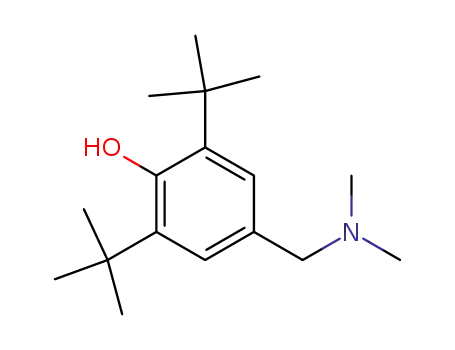
N,N-dimethyl-(3,5-di-tert-butyl-4-hydroxybenzyl)amine
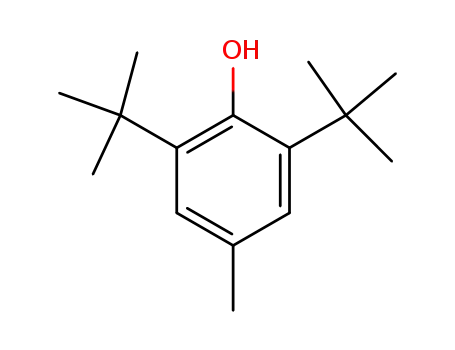
2,6-di-tert-butyl-4-methyl-phenol

Coppinger's Radikal
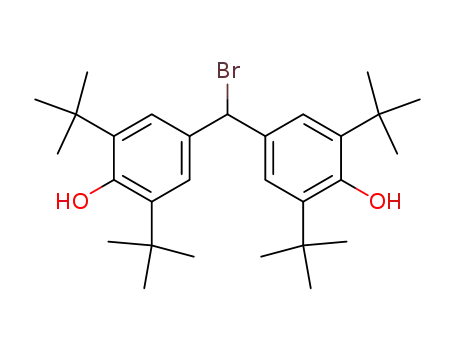
bromo-bis-(3,5-di-tert-butyl-4-hydroxy-phenyl)-methane
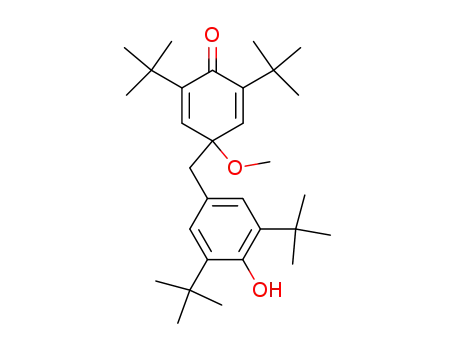
2,6-di-tert-butyl-4-(3,5-di-tert-butyl-4-hydroxy-benzyl)-4-methoxy-cyclohexa-2,5-dienone