- Product Name
- CasNo
- MF
- MW
- Content
- Appearance
- Packing
- Apply
- N-Octyl pyrrolidone
- 2687-94-7
- C12H23NO
- 197.321
Your Location:Home > Products > Electronic Chemicals > N-Octyl pyrrolidone
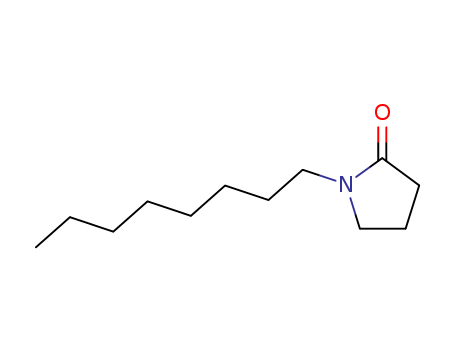

|
Synthesis |
N-Octyl pyrrolidone was synthesized by 2-pyrrolidone and tetrabutylammonium bromide stirred with adding n-octane chloride for 10 h at 90 °C. |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 57, p. 3328, 1992 DOI: 10.1021/jo00038a019 |
|
Flammability and Explosibility |
Nonflammable |
|
Safety Profile |
Moderately toxic by ingestion. Asevere skin and eye irritant. A corrosive. Combustibleliquid. When heated to decomposition it emits toxic fumesof NOx. |
|
General Description |
1-Octyl-2-pyrrolidone is a permeation enhancer and its effect in transport of steroidal permeants across hairless mouse skin was investigated via a parallel pathway skin model. |
InChI:InChI=1/C12H23NO/c1-2-3-4-5-6-7-10-13-11-8-9-12(13)14/h2-11H2,1H3
The selective N-alkylation of amides (cy...
Several N-substituted azacyclopentanones...
The invention relates to a process for t...
Different amides have been selectively m...
Crystallization of active material in sp...
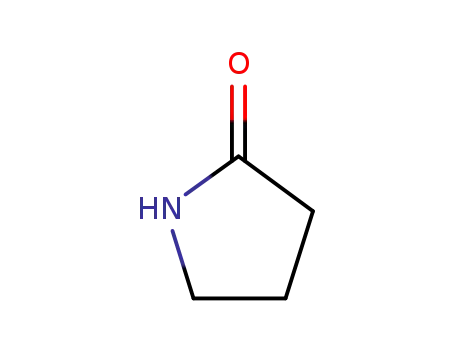
2-pyrrolidinon

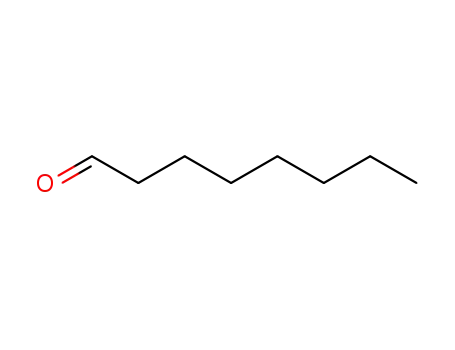
Octanal

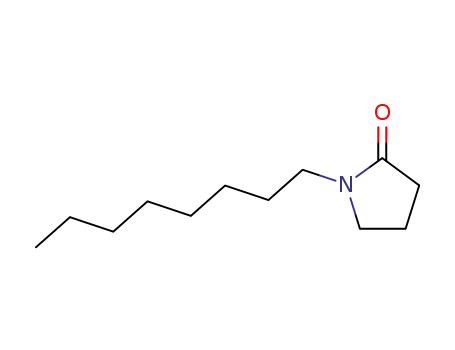
1-n-octyl-2-pyrrolidinone
| Conditions | Yield |
|---|---|
|
With
hydrogen; sodium sulfate;
palladium on activated charcoal;
In
ethyl acetate;
at 100 ℃;
for 4h;
under 30002.4 Torr;
|
93% |
|
With
hydrogen; sodium sulfate;
palladium on activated charcoal;
In
ethyl acetate;
at 100 ℃;
for 4h;
under 30002.4 Torr;
|
93% |

2-pyrrolidinon

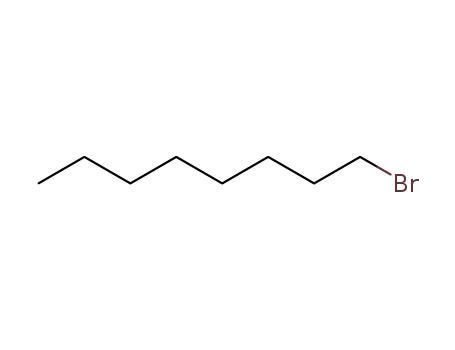
1-bromo-octane


1-n-octyl-2-pyrrolidinone
| Conditions | Yield |
|---|---|
|
With
potassium tert-butylate;
In
dimethyl sulfoxide;
|
85% |
|
With
NaH;
In
toluene; Petroleum ether;
|

2-pyrrolidinon

1-bromo-octane

Octanal
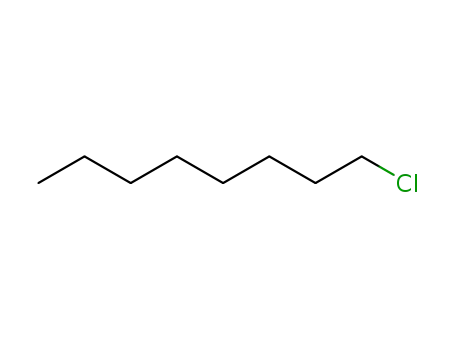
n-chlorooctane
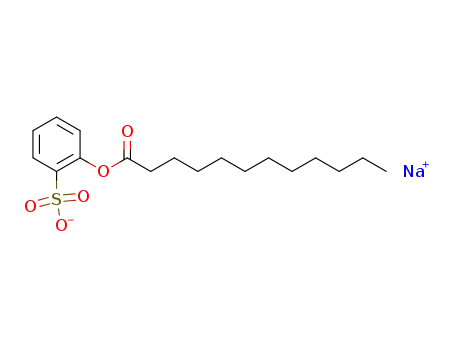
sodium lauroyloxybenzenesulfonate
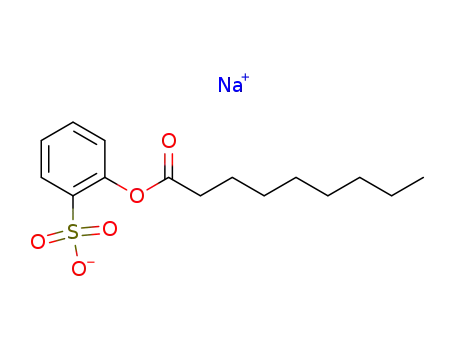
nonanoyloxybenzenesulfonate sodium

2-pyrrolidinon

1-bromo-octane