- Product Name
- CasNo
- MF
- MW
- Content
- Appearance
- Packing
- Apply
- Antioxidant 168
- 31570-04-4
- C42H63O3P
- 646.934
- white crystalline powder
Your Location:Home > Products > Antioxidants > Antioxidant 168
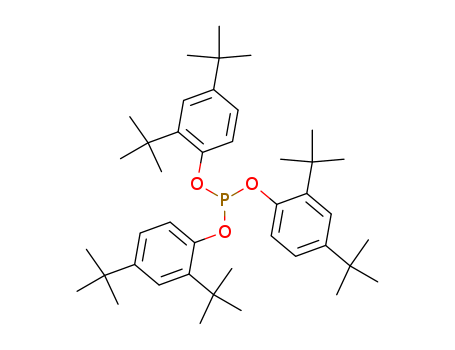

|
Characterization |
Antioxidant 168? is a hydrolytically stable phosphite processing stabilizer. As a secondary antioxidant,Antioxidant 168 reacts during processing with hydroperoxides formed by autoxidation of polymers preventing process induced degradation and extending the performance of primary antioxidants. |
|
Reactions |
Precursor to a palladacyclic catalyst for Suzuki, Stille and Heck processes. Ligand for Pd-catalyzed [3+2] intramolecular cycloaddition of alk-5-enylidenecyclopropanes. Ligand for Pt-catalyzed intramolecular silaboration of alkenes. Ligand for Ni-catalyzed aminocarbonylation of aryl halides. Ligand for the Au-catalyzed [4+2] intramolecular cycloaddition of allene-dienes. Rhodium-Catalyzed Allylic Substitution with an Acyl Anion Equivalent. |
|
Applications |
The application range of Antioxidant 168 -synergistically combined with other Ciba anti-oxidants - comprises polyolefins and olefin-copolymers such as polyethylene (e.g. HDPE, LLDPE), polypropylene, polybutene and ethylene-vinylacetate copolymers as well as polycarbonates and polyamides. The blends can also be used in polyesters, styrene homo- and copolymers, adhesives and natural and synthetic tackifier resins, elastomers such as BR, SEBS, SBS, and other organic substrates. Antioxidant 168? blends can be used in combination with light stabilizers of the TINUVIN and CHIMASSORB range. |
|
Features/benefits |
Antioxidant 168? is an organophosphite of low volatility and is particularly resistant to hydrolysis. It protects polymers which are prone to oxidation, during the processing steps (compounding/ pelletizing, fabrication and recycling) from molecular weight change (e.g. chain scission/crosslinking) and prevents discoloration. Antioxidant 168 performs best when combined with other Ciba antioxidants. Blends of Antioxidant 168 with antioxidants of the IRGANOX range (IRGANOX B-blends) and with Hydroxylamine FS042 are particularly effective. The IRGANOX range antioxidants additionally provide storage stability and give the polymer long term protection against thermo-oxidative degradation. Antioxidant 168 comprised in phenol free systems with other appropriate Ciba stabilizers addresses specific stabilization requirements. |
|
Guidelines for use |
Typically 500 - 2000 ppm of Antioxidant 168 combined with appropriate levels of other additives are used for the processing stabilization of polymers. The optimum level is application specific. Extensive performance data of Antioxidant 168 combinations in various organic polymers and applications are available upon request. |
|
Safety |
In accordance with good industrial practice, handle with care and prevent contamination of the environment. Avoid dust formation and ignition sources. For more detailed information please refer to the material safety data sheet. |
|
Application |
Antioxidant 168 is a kind of phosphite ester antioxidant as processing stabilizer, used for polypropylene, polyethylene, and adhesives. The amount to be used may be 0.1%~1.0% depending on the substrate, processing conditions, and requirements of the end application. Blends with hindered phenols are particularly effective.In addition, they use combination with light stabilizers when need. |
|
General Description |
Tris(2,4-di-tert-butylphenyl) phosphite is a triaryl based phosphite that can be used in catalysis and metallation. Its characteristic to undergo metallation reaction and provide a cost effective synthetic processes allows it to be useful in biaryl coupling reactions. |
|
Flammability and Explosibility |
Notclassified |
|
Properties and Applications |
TEST ITEMS SPECIFICATION APPEARANCE WHITE INCOMPACT POWDER CONTENT 99.0% min MELTING RANGE 183-187 °C VOLATILE 0.3% max SOLUBILITY 2g/20ml TOLUENE CLEAR FREE 2,4-DITERT-BUTYPHENOL 0.2% max (wt) TRANSMITTANCE 98% min 425nm 98% min 500nm ACID VALUE,mg KOH/g 0.3% max HYDROLYSIS TIME (90°C WATER ) 14 h min SOLUBILITY 1% ACETONE 30% TOLUENE 36% CHLOROFORM 4% ETHYL ACETATE 11% n-HEXANE 0.1% ETHANOL 36% DICHLOROETHANE 6% max PHENYLETHYLENE 0.01% max WATER |
|
TEST ITEMS |
SPECIFICATION |
|
APPEARANCE |
WHITE INCOMPACT POWDER |
|
CONTENT |
99.0% min |
|
MELTING RANGE |
183-187 °C |
|
SOLUBILITY 2g/20ml TOLUENE |
CLEAR |
|
FREE 2,4-DITERT-BUTYPHENOL |
0.2% max (wt) |
|
TRANSMITTANCE |
98% min 425nm |
|
ACID VALUE,mg KOH/g |
0.3% max |
|
HYDROLYSIS TIME (90°C WATER ) |
14 h min |
|
SOLUBILITY |
1% ACETONE |
InChI:InChI=1/C14H21O3P/c1-13(2,3)10-7-8-12(17-18(15)16)11(9-10)14(4,5)6/h7-9H,1-6H3/q-2
The invention discloses a preparation me...
The invention provides an organic synthe...
The invention is directed to various alk...
The present invention relates to derivat...
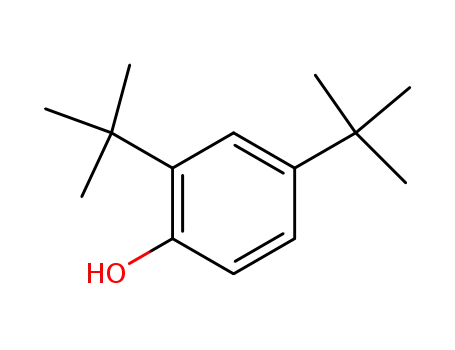
2,4-di-tert-Butylphenol

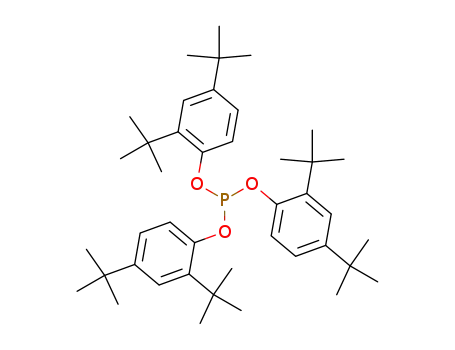
tris(2,4-di-tert-butylphenyl)phosphite
| Conditions | Yield |
|---|---|
|
2,4-di-tert-Butylphenol;
With
pyridine;
In
methanol; toluene;
at 5 - 40 ℃;
With
phosphorus trichloride;
In
methanol; toluene;
at 95 ℃;
for 0.5h;
Reagent/catalyst;
Temperature;
|
98.3% |
|
With
dibutylamine; phosphorus trichloride;
at 55 - 160 ℃;
for 7.5h;
Temperature;
Inert atmosphere;
|
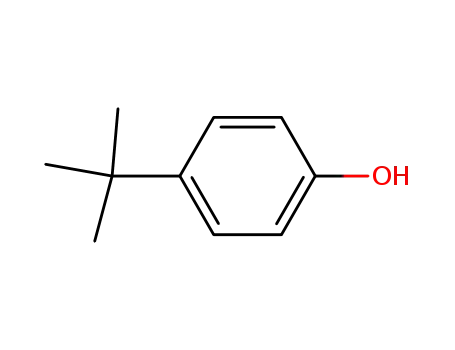
para-tert-butylphenol


2,4-di-tert-Butylphenol


tris(2,4-di-tert-butylphenyl)phosphite

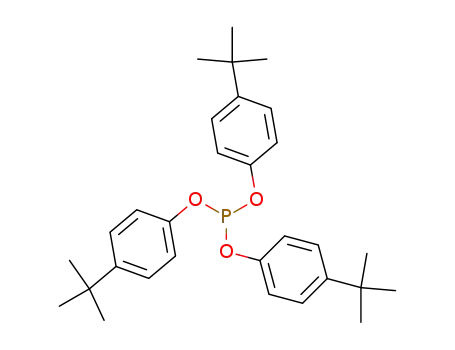
tris(4-(tert-butyl)phenyl) phosphite

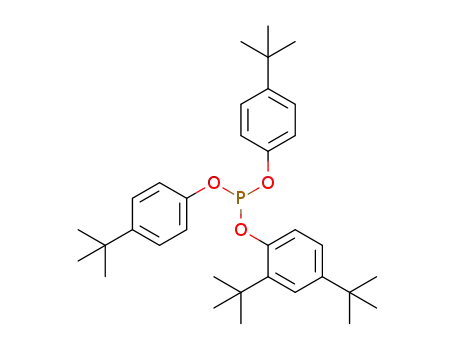
bis(4-tert-butylphenyl)-2,4-di-tert-butylphenyl phosphite

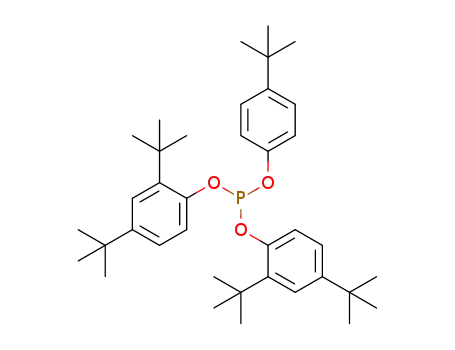
bis(2,4-di-tert-butylphenyl)-4-tert-butylphenyl phosphite
| Conditions | Yield |
|---|---|
|
With
N,N-dimethylaminododecane; phosphorus trichloride;
at 90 - 200 ℃;
under 37.5038 - 750.075 Torr;
Product distribution / selectivity;
Inert atmosphere;
|

para-tert-butylphenol

2,4-di-tert-Butylphenol
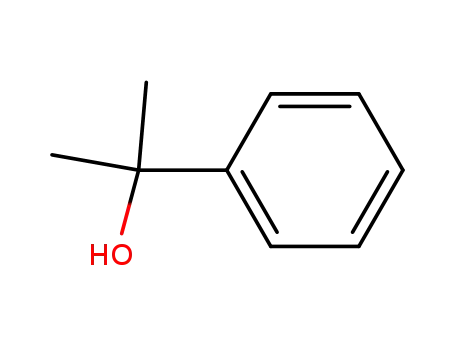
1-methyl-1-phenylethyl alcohol
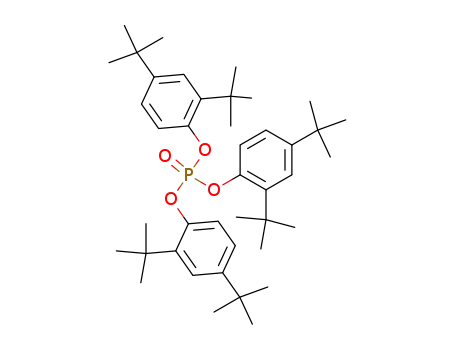
tris(2,4-di(tert-butyl)phenyl) phosphate
2-(2,6-difluorophenyl)-4-(4-ethenylphenyl)oxazoline
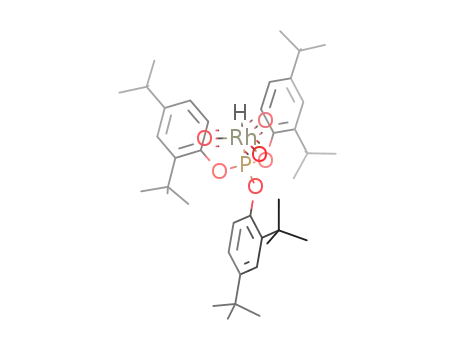
[HRh(CO)3(tris(2,4-di-tert-butylphenyl)phosphite)]